Gap junctions: multifaceted regulators of embryonic cortical development
- PMID: 18403031
- PMCID: PMC2610634
- DOI: 10.1016/j.tins.2008.02.007
Gap junctions: multifaceted regulators of embryonic cortical development
Abstract
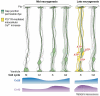
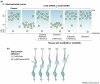
The morphological development of the cerebral cortex from a primitive neuroepithelium into a complex laminar structure underlying higher cognition must rely on a network of intercellular signaling. Gap junctions are widely expressed during embryonic development and provide a means of cell-cell contact and communication. We review the roles of gap junctions in regulating the proliferation of neural progenitors as well as the migration and differentiation of young neurons in the embryonic cerebral cortex. There is substantial evidence that although gap junctions act in the classical manner coupling neural progenitors, they also act as hemichannels mediating the spread of calcium waves across progenitor cell populations and as adhesive molecules aiding neuronal migration. Gap junctions are thus emerging as multifaceted regulators of cortical development playing diverse roles in intercellular communication.
Figures


References
-
- Kriegstein A, et al. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 2006;7:883–890. - PubMed
-
- Lo CW. The role of gap junction membrane channels in development. J. Bioenerg. Biomembr. 1996;28:379–385. - PubMed
-
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

