What are comparative studies telling us about the mechanism of ERbeta action in the ERE-dependent E2 signaling pathway?
- PMID: 18403199
- PMCID: PMC2577834
- DOI: 10.1016/j.jsbmb.2008.03.001
What are comparative studies telling us about the mechanism of ERbeta action in the ERE-dependent E2 signaling pathway?
Abstract
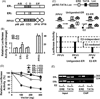
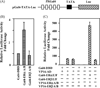
Estrogen hormone (E2) signaling is primarily conveyed by the estrogen receptors (ER) alpha and beta. ERs are encoded by two distinct genes and share varying degrees of domain-specific structural/functional similarities. ERs mediate a complex array of nuclear and non-nuclear events critical for the homeodynamic regulation of various tissue functions. The canonical nuclear signaling involves the interaction of ERalpha and ERbeta with specific DNA sequences, the so-called estrogen responsive elements (EREs). This interaction constitutes the initial step in ERE-dependent signaling in which ERbeta is a weaker transcription factor than ERalpha in response to E2. However, it remains unclear why transactivation potencies of ER subtypes differ. Studies suggest that the amino-terminus, the least conserved structural region, of ERbeta, but not that of ERalpha, impairs the ability of the receptor to bind to ERE independent of E2. Although the impaired ERbeta-ERE interaction contributes, it is not sufficient to explain the weak transactivation potency of the receptor. It appears that the lack of transactivation ability and of the capability of the amino-terminus of ERbeta, as opposed to that of ERalpha, to functionally interact with the carboxyl-terminal hormone-dependent activation domain is also critical for the receptor-specific activity. Thus, the structurally distinct amino-termini of ERs are important determinants in defining the function of ER-subtypes in the ERE-dependent pathway. This could differentially affect the physiology and pathophysiology of E2 signaling.
Figures

Similar articles
-
Binding of estrogen receptor beta to estrogen response element in situ is independent of estradiol and impaired by its amino terminus.Mol Endocrinol. 2005 Nov;19(11):2696-712. doi: 10.1210/me.2005-0120. Epub 2005 Jun 23. Mol Endocrinol. 2005. PMID: 15976006
-
Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways.Mol Cell Biol. 2004 Sep;24(17):7681-94. doi: 10.1128/MCB.24.17.7681-7694.2004. Mol Cell Biol. 2004. PMID: 15314175 Free PMC article.
-
Genomic responses from the estrogen-responsive element-dependent signaling pathway mediated by estrogen receptor alpha are required to elicit cellular alterations.J Biol Chem. 2009 May 29;284(22):15277-88. doi: 10.1074/jbc.M900365200. Epub 2009 Mar 24. J Biol Chem. 2009. PMID: 19321454 Free PMC article.
-
Xenoestrogen regulation of ERα/ERβ balance in hormone-associated cancers.Mol Cell Endocrinol. 2017 Dec 5;457:3-12. doi: 10.1016/j.mce.2016.10.033. Epub 2016 Nov 2. Mol Cell Endocrinol. 2017. PMID: 27816767 Review.
-
The extra-nuclear interactome of the estrogen receptors: implications for physiological functions.Mol Cell Endocrinol. 2021 Dec 1;538:111452. doi: 10.1016/j.mce.2021.111452. Epub 2021 Sep 7. Mol Cell Endocrinol. 2021. PMID: 34500041 Review.
Cited by
-
Absolute Quantification of Phosphorylated ERβ Amino Acids in the Hippocampus of Women and in A Rat Model of Menopause.Endocrinology. 2021 Sep 1;162(9):bqab122. doi: 10.1210/endocr/bqab122. Endocrinology. 2021. PMID: 34147032 Free PMC article.
-
Molecular mechanism of estrogen-estrogen receptor signaling.Reprod Med Biol. 2016 Dec 5;16(1):4-20. doi: 10.1002/rmb2.12006. eCollection 2017 Jan. Reprod Med Biol. 2016. PMID: 29259445 Free PMC article. Review.
-
Estrogen receptors similarly mediate the effects of 17β-estradiol on cellular responses but differ in their potencies.Endocrine. 2011 Feb;39(1):48-61. doi: 10.1007/s12020-010-9411-8. Epub 2010 Nov 11. Endocrine. 2011. PMID: 21069581 Free PMC article.
-
Estrogen Receptor Function: Impact on the Human Endometrium.Front Endocrinol (Lausanne). 2022 Feb 28;13:827724. doi: 10.3389/fendo.2022.827724. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35295981 Free PMC article. Review.
-
Do Estrogen Receptor beta Polymorphisms Play A Role in the Pharmacogenetics of Estrogen Signaling?Curr Pharmacogenomics Person Med. 2008 Dec 1;6(4):239-259. doi: 10.2174/187569208786733820. Curr Pharmacogenomics Person Med. 2008. PMID: 19337586 Free PMC article.
References
-
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. - PubMed
-
- Nilsson S, Gustafsson JA. Estrogen receptor action. Crit Rev Eukaryot Gene Expr. 2002;12:237–257. - PubMed
-
- Yi P, Driscoll MD, Huang J, Bhagat S, Hilf R, Bambara RA, Muyan M. The effects of estrogen-responsive element- and ligand-induced structural changes on the recruitment of cofactors and transcriptional responses by ERα and ERβ. Mol Endocrinol. 2002;16:674–693. - PubMed
-
- Loven MA, Wood JR, Nardulli AM. Interaction of estrogen receptors α and β with estrogen response elements. Mol Cell Endocrinol. 2001;181:151–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

