EphB receptors co-distribute with a nicotinic receptor subtype and regulate nicotinic downstream signaling in neurons
- PMID: 18403216
- PMCID: PMC2459225
- DOI: 10.1016/j.mcn.2008.02.013
EphB receptors co-distribute with a nicotinic receptor subtype and regulate nicotinic downstream signaling in neurons
Abstract
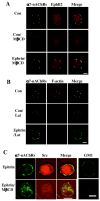
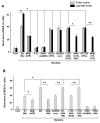
Activation of nicotinic acetylcholine receptors (nAChRs) on neurons engages calcium-dependent signaling pathways regulating numerous events. Receptors containing alpha7 subunits (alpha7-nAChRs) are prominent in this because of their abundance and high relative calcium permeability. We show here that EphB2 receptors are co-localized with postsynaptic alpha7-nAChRs on chick ciliary ganglion neurons and that treatment of the cells with an ephrinB1 construct to activate the EphB receptors exerts physical restraints on both classes of receptors, diminishing their dispersal after spine retraction or lipid raft disruption. Moreover, the ephrinB1/EphB receptor complex specifically enhances the ability of alpha7-nAChRs to activate the transcription factor CREB, acting through a pathway including a receptor tyrosine kinase, a Src family member, PI3 kinase, and protein kinase A most distally. The enhancement does not appear to result from a change in the alpha7-nAChR current amplitude, suggesting a downstream target. The results demonstrate a role for ephrin/EphB action in nicotinic signaling.
Figures




Similar articles
-
Reversible inhibition of GABAA receptors by alpha7-containing nicotinic receptors on the vertebrate postsynaptic neurons.J Physiol. 2007 Mar 15;579(Pt 3):753-63. doi: 10.1113/jphysiol.2006.124578. Epub 2007 Jan 4. J Physiol. 2007. PMID: 17204496 Free PMC article.
-
Brain-derived neurotrophic factor and trkB signaling in parasympathetic neurons: relevance to regulating alpha7-containing nicotinic receptors and synaptic function.J Neurosci. 2004 May 5;24(18):4340-50. doi: 10.1523/JNEUROSCI.0055-04.2004. J Neurosci. 2004. PMID: 15128848 Free PMC article.
-
[Suppression of nicotinic ACh receptors-mediated currents by activation of Eph/Ephrin-B1 signaling involves Src tyrosine kinase and mitogen-activated protein kinase in ciliary ganglion neurons].Sheng Li Xue Bao. 2008 Aug 25;60(4):462-8. Sheng Li Xue Bao. 2008. PMID: 18690387 Chinese.
-
Role of the α7 Nicotinic Acetylcholine Receptor and RIC-3 in the Cholinergic Anti-inflammatory Pathway.Cent Nerv Syst Agents Med Chem. 2017;17(2):90-99. doi: 10.2174/1871524916666160829114533. Cent Nerv Syst Agents Med Chem. 2017. PMID: 27573666 Review.
-
The alpha7 nicotinic acetylcholine receptor in neuronal plasticity.Mol Neurobiol. 1999 Aug;20(1):1-16. doi: 10.1007/BF02741361. Mol Neurobiol. 1999. PMID: 10595869 Review.
Cited by
-
Modulatory effects of α7 nAChRs on the immune system and its relevance for CNS disorders.Cell Mol Life Sci. 2016 Jul;73(13):2511-30. doi: 10.1007/s00018-016-2175-4. Epub 2016 Mar 15. Cell Mol Life Sci. 2016. PMID: 26979166 Free PMC article. Review.
-
Lateral mobility of nicotinic acetylcholine receptors on neurons is determined by receptor composition, local domain, and cell type.J Neurosci. 2010 Jun 30;30(26):8841-51. doi: 10.1523/JNEUROSCI.6236-09.2010. J Neurosci. 2010. PMID: 20592206 Free PMC article.
-
Critical time window of neuronal cholesterol synthesis during neurite outgrowth.J Neurosci. 2012 May 30;32(22):7632-45. doi: 10.1523/JNEUROSCI.1352-11.2012. J Neurosci. 2012. PMID: 22649242 Free PMC article.
-
Multiple cell adhesion molecules shaping a complex nicotinic synapse on neurons.Mol Cell Neurosci. 2008 Sep;39(1):74-82. doi: 10.1016/j.mcn.2008.05.017. Epub 2008 Jun 4. Mol Cell Neurosci. 2008. PMID: 18585463 Free PMC article.
-
Cortical synaptic NMDA receptor deficits in α7 nicotinic acetylcholine receptor gene deletion models: implications for neuropsychiatric diseases.Neurobiol Dis. 2014 Mar;63:129-40. doi: 10.1016/j.nbd.2013.11.021. Epub 2013 Dec 8. Neurobiol Dis. 2014. PMID: 24326163 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

