Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model
- PMID: 18403224
- PMCID: PMC2430082
- DOI: 10.1016/j.prostaglandins.2008.02.005
Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model
Abstract
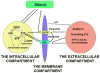
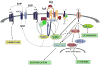
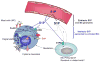
Sphingosine-1-phosphate (S1P) is a lipid mediator involved in diverse biological processes, from vascular and neural development to the regulation of lymphocyte trafficking. Many of its functions are regulated by five widely expressed S1P G-protein-coupled receptors (S1P(1-5)). S1P is produced mostly intracellularly, thus, much of its potential as an autocrine and paracrine mediator depends on how, when, and where it is generated or secreted out of the cells. However, S1P can also have intracellular activity independent of its receptors, adding to the complexity of S1P function. The mast cell, a major effector cell during an allergic response, has proven instrumental towards understanding the complex regulation and function of S1P. Antigen (Ag) engagement of the IgE receptor in mast cells stimulates sphingosine kinases, which generate S1P and are involved in the activation of calcium fluxes critical for mast cell responses. In addition, mast cells secrete considerable amounts of S1P upon activation, thus affecting the surrounding tissues and recruiting inflammatory cells. Export of S1P is also involved in the autocrine transactivation of S1P receptors present in mast cells. The in vivo response of mast cells, however, is not strictly dependent on their ability to generate S1P, but they are also affected by changes in S1P in the environment previous to Ag challenge. This review will discuss the recent advances towards understanding the intricacies of S1P generation, secretion and regulation in mast cells. In addition, how S1P receptors are activated and their involvement in mast cell functions will also be covered, including new insights on the role of S1P in the mast cell-mediated allergic response of systemic anaphylaxis.
Figures

Similar articles
-
The sphingosine-1-phosphate/sphingosine-1-phosphate receptor 2 axis regulates early airway T-cell infiltration in murine mast cell-dependent acute allergic responses.J Allergy Clin Immunol. 2015 Apr;135(4):1008-1018.e1. doi: 10.1016/j.jaci.2014.10.044. Epub 2014 Dec 13. J Allergy Clin Immunol. 2015. PMID: 25512083 Free PMC article.
-
The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis.Immunity. 2007 Mar;26(3):287-97. doi: 10.1016/j.immuni.2007.02.008. Epub 2007 Mar 8. Immunity. 2007. PMID: 17346996
-
Export and functions of sphingosine-1-phosphate.Biochim Biophys Acta. 2009 Jul;1791(7):692-6. doi: 10.1016/j.bbalip.2009.02.011. Epub 2009 Mar 4. Biochim Biophys Acta. 2009. PMID: 19268560 Free PMC article. Review.
-
An emerging role for the lipid mediator sphingosine-1-phosphate in mast cell effector function and allergic disease.Adv Exp Med Biol. 2011;716:123-42. doi: 10.1007/978-1-4419-9533-9_8. Adv Exp Med Biol. 2011. PMID: 21713655 Free PMC article. Review.
-
Role of sphingosine-1-phosphate (S1P) and the S1P(2) receptor in allergen-induced, mast cell-dependent contraction of rat lung parenchymal strips.Naunyn Schmiedebergs Arch Pharmacol. 2009 Oct;380(4):303-9. doi: 10.1007/s00210-009-0438-4. Epub 2009 Jul 28. Naunyn Schmiedebergs Arch Pharmacol. 2009. PMID: 19636535
Cited by
-
Shaping the landscape: metabolic regulation of S1P gradients.Biochim Biophys Acta. 2013 Jan;1831(1):193-202. doi: 10.1016/j.bbalip.2012.06.007. Epub 2012 Jun 23. Biochim Biophys Acta. 2013. PMID: 22735358 Free PMC article. Review.
-
The role of sphingosine-1-phosphate transporter Spns2 in immune system function.J Immunol. 2012 Jul 1;189(1):102-11. doi: 10.4049/jimmunol.1200282. Epub 2012 Jun 4. J Immunol. 2012. PMID: 22664872 Free PMC article.
-
Role of Bioactive Sphingolipids in Inflammation and Eye Diseases.Adv Exp Med Biol. 2019;1161:149-167. doi: 10.1007/978-3-030-21735-8_14. Adv Exp Med Biol. 2019. PMID: 31562629 Free PMC article.
-
Usage of sphingosine kinase isoforms in mast cells is species and/or cell type determined.J Immunol. 2013 Mar 1;190(5):2058-67. doi: 10.4049/jimmunol.1201503. Epub 2013 Jan 28. J Immunol. 2013. PMID: 23359503 Free PMC article.
-
Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema.J Exp Med. 2010 Mar 15;207(3):465-74. doi: 10.1084/jem.20091513. Epub 2010 Mar 1. J Exp Med. 2010. PMID: 20194630 Free PMC article.
References
-
- Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. - PubMed
-
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. - PubMed
-
- Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. - PubMed
-
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. - PubMed
-
- Yatomi Y. Sphingosine 1-phosphate in vascular biology: possible therapeutic strategies to control vascular diseases. Curr Pharm Des. 2006;12:575–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

