Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation
- PMID: 18403318
- PMCID: PMC2431108
- DOI: 10.1194/jlr.M700537-JLR200
Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation
Abstract
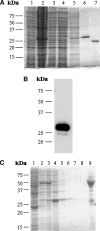
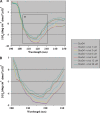
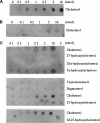
StarD4 protein is a member of the StarD4 subfamily of steroidogenic acute regulatory-related lipid transfer (START) domain proteins that includes StarD5 and StarD6, proteins whose functions remain poorly defined. The objective of this study was to isolate and characterize StarD4's sterol binding and to determine in a hepatocyte culture model its sterol transport capabilities. Utilizing purified full-length StarD4, in vitro binding assays demonstrated a concentration-dependent binding of [(14)C]cholesterol by StarD4 similar to that of the cholesterol binding START domain proteins StarD1 and StarD5. Other tested sterols showed no detectable binding to StarD4, except for 7alpha-hydroxycholesterol, for which StarD4 demonstrated weak binding on lipid protein overlay assays. Subsequently, an isolated mouse hepatocyte model was used to study the ability of StarD4 to bind/mobilize/distribute cellular cholesterol. Increased expression of StarD4 in primary mouse hepatocytes led to a marked increase in the intracellular cholesteryl ester concentration and in the rates of bile acid synthesis. The ability and specificity of StarD4 to bind cholesterol and, as a function of its level of expression, to direct endogenous cellular cholesterol suggest that StarD4 plays an important role as a directional cholesterol transporter in the maintenance of cellular cholesterol homeostasis.
Figures



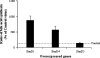



Similar articles
-
Human StarD5, a cytosolic StAR-related lipid binding protein.J Lipid Res. 2005 Aug;46(8):1615-23. doi: 10.1194/jlr.M400501-JLR200. Epub 2005 May 16. J Lipid Res. 2005. PMID: 15897605
-
Differential gene regulation of StarD4 and StarD5 cholesterol transfer proteins. Activation of StarD4 by sterol regulatory element-binding protein-2 and StarD5 by endoplasmic reticulum stress.J Biol Chem. 2005 May 13;280(19):19410-8. doi: 10.1074/jbc.M501778200. Epub 2005 Mar 10. J Biol Chem. 2005. PMID: 15760897
-
The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6.Proc Natl Acad Sci U S A. 2002 May 14;99(10):6943-8. doi: 10.1073/pnas.052143799. Proc Natl Acad Sci U S A. 2002. PMID: 12011452 Free PMC article.
-
The StarD4 subfamily of steroidogenic acute regulatory-related lipid transfer (START) domain proteins: new players in cholesterol metabolism.Int J Biochem Cell Biol. 2014 Apr;49:64-8. doi: 10.1016/j.biocel.2014.01.002. Epub 2014 Jan 15. Int J Biochem Cell Biol. 2014. PMID: 24440759 Free PMC article. Review.
-
The binding site specificity of STARD4 subfamily: Breaking the cholesterol paradigm.Mol Cell Endocrinol. 2015 Jun 15;408:53-61. doi: 10.1016/j.mce.2014.12.016. Epub 2014 Dec 24. Mol Cell Endocrinol. 2015. PMID: 25542846 Review.
Cited by
-
Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones.Nutr Metab (Lond). 2010 Jun 1;7:47. doi: 10.1186/1743-7075-7-47. Nutr Metab (Lond). 2010. PMID: 20515451 Free PMC article.
-
Early steps in steroidogenesis: intracellular cholesterol trafficking.J Lipid Res. 2011 Dec;52(12):2111-2135. doi: 10.1194/jlr.R016675. Epub 2011 Oct 5. J Lipid Res. 2011. PMID: 21976778 Free PMC article. Review.
-
StAR-related lipid transfer domain protein 5 binds primary bile acids.J Lipid Res. 2012 Dec;53(12):2677-89. doi: 10.1194/jlr.M031245. Epub 2012 Sep 26. J Lipid Res. 2012. PMID: 23018617 Free PMC article.
-
MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein.J Lipid Res. 2010 May;51(5):1023-34. doi: 10.1194/jlr.M002345. Epub 2009 Oct 29. J Lipid Res. 2010. PMID: 19965586 Free PMC article.
-
STARTing to understand MLN64 function in cholesterol transport.J Lipid Res. 2010 Aug;51(8):2015-7. doi: 10.1194/jlr.E008854. Epub 2010 May 28. J Lipid Res. 2010. PMID: 20511492 Free PMC article. No abstract available.
References
-
- Hylemon, P. B., W. M. Pandak, and Z. R. Vlahcevic. 2001. The liver: biology and pathobiology. In The Liver. I. M. Arias, J. L. Boyer, F. V. Chisari, et al., editors. Lippincott Williams & Wilkins, Philadelphia, PA. 231–247.
-
- Strauss J. F., III, T. Kishida, L. K. Christenson, T. Fujimoto, and H. Hiroi. 2003. START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells. Mol. Cell. Endocrinol. 202 59–65. - PubMed
-
- Ponting C. P., and L. Aravind. 2004. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem. Sci. 24 130–132. - PubMed
-
- Rodriguez-Agudo D., S. Ren, P. Hylemon, K. Redford, R. Natarajan, A. Del Castillo, G. Gil, and W. Pandak. 2005. Human StarD5, a cytosolic StAR-related lipid binding protein. J. Lipid Res. 46 1615–1623. - PubMed
-
- Iyer L. M., E. V. Koonin, and L. Aravind. 2001. Adaptations of the helix-grip fold for ligand binding and catalysis in the START domain superfamily. Proteins. 43 134–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

