Two different forms of long-term potentiation at CA1-subiculum synapses
- PMID: 18403426
- PMCID: PMC2536578
- DOI: 10.1113/jphysiol.2007.149203
Two different forms of long-term potentiation at CA1-subiculum synapses
Abstract
Distinct functional roles in learning and memory are attributed to certain areas of the hippocampus and the parahippocampal region. The subiculum as a part of the hippocampal formation is the principal target of CA1 pyramidal cell axons and serves as an interface in the information processing between the hippocampus and the neocortex. Subicular pyramidal cells have been classified as bursting and regular firing cells. Here we report fundamental differences in long-term potentiation (LTP) between both cell types. Prolonged high-frequency stimulation induced NMDA receptor-dependent LTP in both cell types. While LTP relied on postsynaptic calcium in regular firing neurons, no increase in postsynaptic calcium was required in bursting cells. Furthermore, paired-pulse facilitation revealed that the site of LTP expression was postsynaptic in regular firing neurons, while presynaptic in burst firing neurons. Our findings on synaptic plasticity in the subiculum indicate that regular firing and bursting cells represent two functional units with distinct physiological roles in processing hippocampal output.
Figures




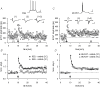




References
-
- Amaral DG, Witter MP. The hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. New York: Academic Press; 1995. pp. 443–494.
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Boeijinga PH, Boddeke HW. Activation of 5-HT1B receptors suppresses low but not high frequency synaptic transmission in the rat subicular cortex in vitro. Brain Res. 1996;721:59–65. - PubMed
-
- Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33:123–130. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

