Glucocorticoids induce human glycoprotein hormone alpha-subunit gene expression in the gonadotrope
- PMID: 18403486
- PMCID: PMC2453092
- DOI: 10.1210/en.2007-1100
Glucocorticoids induce human glycoprotein hormone alpha-subunit gene expression in the gonadotrope
Abstract
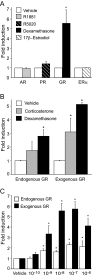
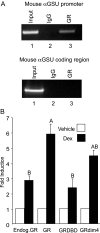
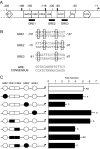
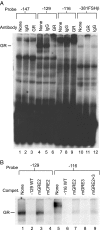
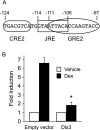
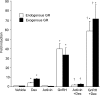
The human glycoprotein hormone alpha-subunit (alphaGSU) gene is transcriptionally regulated by glucocorticoids in a cell type-specific fashion. In direct contrast to repression of alphaGSU by glucocorticoids in placenta, glucocorticoid receptor (GR) modulation in the pituitary is little understood. We show that glucocorticoids stimulate the alphaGSU promoter in immortalized pituitary gonadotrope-derived LbetaT2 cells, whereas estrogens, androgens, and progestins have no significant effect. Moreover, GR acts in a dose-dependent manner at physiological concentrations of glucocorticoids. Transient transfection of GR with dexamethasone (Dex) treatment further stimulates the alphaGSU promoter, but this induction is severely diminished using a receptor mutated in the DNA-binding domain. Truncation and cis mutations demonstrate that glucocorticoid response element 2 (GRE2) and cAMP-response element 2 (CRE2) within -168 bp of the human alphaGSU promoter are critical for induction. Moreover, dominant-negative CRE-binding protein markedly inhibits basal but also Dex induction of alphaGSU promoter activity. Additionally, GR specifically binds to GRE2 in the human alphaGSU promoter in vitro and to the 5' region of the endogenous mouse alphaGSU gene in vivo. Furthermore, overexpression of the homeobox factor, Distal-less 3 that regulates this gene in placental cells through a site partially overlapping GRE2, blocks Dex induction of alphaGSU in gonadotrope cells, indicating that placenta-specific expression of Dlx3 may interfere with GR, resulting in repression in placental cells vs. induction in gonadotrope cells. These results demonstrate the stimulatory role played by glucocorticoids in alphaGSU gene expression in the pituitary gonadotrope, in contrast to repression in placental cells, and highlight the tissue-specific nature of steroid hormone action.
Figures


Similar articles
-
Msx1 homeodomain protein represses the αGSU and GnRH receptor genes during gonadotrope development.Mol Endocrinol. 2013 Mar;27(3):422-36. doi: 10.1210/me.2012-1289. Epub 2013 Jan 31. Mol Endocrinol. 2013. PMID: 23371388 Free PMC article.
-
Regulation of human glycoprotein hormone alpha-subunit gene transcription in LbetaT2 gonadotropes by protein kinase C and extracellular signal-regulated kinase 1/2.Biol Reprod. 2002 Sep;67(3):725-34. doi: 10.1095/biolreprod67.3.725. Biol Reprod. 2002. PMID: 12193378
-
Repression of the human glycoprotein hormone alpha-subunit gene by glucocorticoids: evidence for receptor interactions with limiting transcriptional activators.Mol Endocrinol. 1991 Jan;5(1):100-10. doi: 10.1210/mend-5-1-100. Mol Endocrinol. 1991. PMID: 1708098
-
Steroidogenic factor-1 and the gonadotrope-specific element enhance basal and pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein hormone alpha-subunit gene in gonadotropes.Mol Endocrinol. 2003 Nov;17(11):2177-88. doi: 10.1210/me.2002-0393. Epub 2003 Aug 14. Mol Endocrinol. 2003. PMID: 12920232
-
Glucocorticoid receptor cofactors as therapeutic targets.Curr Opin Pharmacol. 2010 Dec;10(6):613-9. doi: 10.1016/j.coph.2010.08.001. Epub 2010 Aug 26. Curr Opin Pharmacol. 2010. PMID: 20801081 Free PMC article. Review.
Cited by
-
Msx1 homeodomain protein represses the αGSU and GnRH receptor genes during gonadotrope development.Mol Endocrinol. 2013 Mar;27(3):422-36. doi: 10.1210/me.2012-1289. Epub 2013 Jan 31. Mol Endocrinol. 2013. PMID: 23371388 Free PMC article.
-
Stress levels of glucocorticoids inhibit LHβ-subunit gene expression in gonadotrope cells.Mol Endocrinol. 2012 Oct;26(10):1716-31. doi: 10.1210/me.2011-1327. Epub 2012 Jul 31. Mol Endocrinol. 2012. PMID: 22851703 Free PMC article.
-
Influence of stress-induced intermediates on gonadotropin gene expression in gonadotrope cells.Mol Cell Endocrinol. 2014 Mar 25;385(1-2):71-7. doi: 10.1016/j.mce.2013.08.014. Epub 2013 Sep 4. Mol Cell Endocrinol. 2014. PMID: 24012628 Free PMC article. Review.
-
Androgen receptor drives transcription of rat PACAP in gonadotrope cells.Mol Endocrinol. 2013 Aug;27(8):1343-56. doi: 10.1210/me.2012-1378. Epub 2013 Jun 24. Mol Endocrinol. 2013. PMID: 23798575 Free PMC article.
-
Pituitary Tumors and Immortalized Cell Lines Generated by Cre-Inducible Expression of SV40 T Antigen.Endocrinology. 2021 Jul 1;162(7):bqab073. doi: 10.1210/endocr/bqab073. Endocrinology. 2021. PMID: 33837405 Free PMC article.
References
-
- Gharib SD, Wierman ME, Shupnik MA, Chin WW 1990 Molecular biology of the pituitary gonadotropins. Endocr Rev 11:177–199 - PubMed
-
- Fiddes JC, Goodman HM 1981 The gene encoding the common α-subunit of the four human glycoprotein hormones. J Mol Appl Genet 1:3–18 - PubMed
-
- Shupnik MA 1996 Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol Reprod 54:279–286 - PubMed
-
- Keri RA, Andersen B, Kennedy GC, Hamernik DL, Clay CM, Brace AD, Nett TM, Notides AC, Nilson JH 1991 Estradiol inhibits transcription of the human glycoprotein hormone α-subunit gene despite the absence of a high affinity binding site for estrogen receptor. Mol Endocrinol 5:725–733 - PubMed
-
- Dalkin AC, Haisenleder DJ, Ortolano GA, Suhr A, Marshall JC 1990 Gonadal regulation of gonadotropin subunit gene expression: evidence for regulation of follicle-stimulating hormone-β messenger ribonucleic acid by nonsteroidal hormones in female rats. Endocrinology 127:798–806 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

