Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen
- PMID: 18403489
- PMCID: PMC2592075
- DOI: 10.1210/en.2008-0086
Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen
Abstract
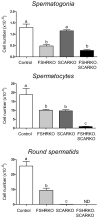
Spermatogenesis in the adult male depends on the action of FSH and androgen. Ablation of either hormone has deleterious effects on Sertoli cell function and the progression of germ cells through spermatogenesis. In this study we generated mice lacking both FSH receptors (FSHRKO) and androgen receptors on the Sertoli cell (SCARKO) to examine how FSH and androgen combine to regulate Sertoli cell function and spermatogenesis. Sertoli cell number in FSHRKO-SCARKO mice was reduced by about 50% but was not significantly different from FSHRKO mice. In contrast, total germ cell number in FSHRKO-SCARKO mice was reduced to 2% of control mice (and 20% of SCARKO mice) due to a failure to progress beyond early meiosis. Measurement of Sertoli cell-specific transcript levels showed that about a third were independent of hormonal action on the Sertoli cell, whereas others were predominantly androgen dependent or showed redundant control by FSH and androgen. Results show that FSH and androgen act through redundant, additive, and synergistic regulation of spermatogenesis and Sertoli cell activity. In addition, the Sertoli cell retains a significant capacity for activity, which is independent of direct hormonal regulation.
Figures



References
-
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. - PubMed
-
- Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. - PubMed
-
- McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179. - PubMed
-
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. - PubMed
-
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

