Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors
- PMID: 18403491
- PMCID: PMC2563054
- DOI: 10.1215/15228517-2008-012
Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors
Abstract
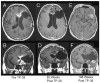
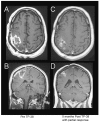
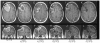
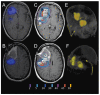
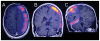
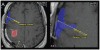
The purpose of this study is to determine the maximum tolerated dose (MTD), dose-limiting toxicity (DLT), and intracerebral distribution of a recombinant toxin (TP-38) targeting the epidermal growth factor receptor in patients with recurrent malignant brain tumors using the intracerebral infusion technique of convection-enhanced delivery (CED). Twenty patients were enrolled and stratified for dose escalation by the presence of residual tumor from 25 to 100 ng/ml in a 40-ml infusion volume. In the last eight patients, coinfusion of (123)I-albumin was performed to monitor distribution within the brain. The MTD was not reached in this study. Dose escalation was stopped at 100 ng/ml due to inconsistent drug delivery as evidenced by imaging the coinfused (123)I-albumin. Two DLTs were seen, and both were neurologic. Median survival after TP-38 was 28 weeks (95% confidence interval, 26.5-102.8). Of 15 patients treated with residual disease, two (13.3%) demonstrated radiographic responses, including one patient with glioblastoma multiforme who had a nearly complete response and remains alive >260 weeks after therapy. Coinfusion of (123)I-albumin demonstrated that high concentrations of the infusate could be delivered >4 cm from the catheter tip. However, only 3 of 16 (19%) catheters produced intraparenchymal infusate distribution, while the majority leaked infusate into the cerebrospinal fluid spaces. Intracerebral CED of TP-38 was well tolerated and produced some durable radiographic responses at doses <or=100 ng/ml. CED has significant potential for enhancing delivery of therapeutic macromolecules throughout the human brain. However, the potential efficacy of drugs delivered by this technique may be severely constrained by ineffective infusion in many patients.
Figures
References
-
- CBTRUS, Central Brain Tumor Registry of the United States. Statistical Report: Primary Brain Tumors in the United States, 1997–2001. Chicago: CBTRUS; 2004.
-
- Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–822. - PubMed
-
- Pai LH, Pastan I. Immunotoxins and recombinant toxins. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Biologic Therapy of Cancer. Philadelphia: J.B. Lippincott; 1995. pp. 521–533.
-
- Phillips PC, Levow C, Catterall M, et al. Transforming growth factor-alpha-Pseudomonas exotoxin fusion protein (TGF-alpha-PE38) treatment of subcutaneous and intracranial human glioma and medulloblastoma xenografts in athymic mice. Cancer Res. 1994;54:1008–1015. - PubMed
-
- Pastan I. Targeted therapy of cancer with recombinant immunotoxins. Biochimica et Biophysica Acta. 1997;1333:C1–C6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

