Deconstruction of iterative multidomain polyketide synthase function
- PMID: 18403714
- PMCID: PMC2480491
- DOI: 10.1126/science.1154711
Deconstruction of iterative multidomain polyketide synthase function
Abstract
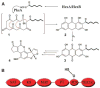
PksA, which initiates biosynthesis of the environmental carcinogen aflatoxin B1, is one of the multidomain iterative polyketide synthases (IPKSs), a large, poorly understood family of biosynthetic enzymes. We found that dissection of PksA and its reconstitution from selected sets of domains allows the accumulation and characterization of advanced octaketide intermediates bound to the enzyme, permitting the reactions controlled by individual catalytic domains to be identified. A product template (PT) domain unites with the ketosynthase and thioesterase in this IPKS system to assemble precisely seven malonyl-derived building blocks to a hexanoyl starter unit and mediate a specific cyclization cascade. Because the PT domain is common among nonreducing IPKSs, these mechanistic features should prove to be general for IPKS-catalyzed production of aromatic polyketides.
Figures


Comment in
-
Biochemistry. Anatomy of a fungal polyketide synthase.Science. 2008 Apr 11;320(5873):186-7. doi: 10.1126/science.1157677. Science. 2008. PMID: 18403699 No abstract available.
Similar articles
-
The architectures of iterative type I PKS and FAS.Nat Prod Rep. 2018 Oct 17;35(10):1046-1069. doi: 10.1039/c8np00039e. Nat Prod Rep. 2018. PMID: 30137093 Free PMC article. Review.
-
Structural basis for biosynthetic programming of fungal aromatic polyketide cyclization.Nature. 2009 Oct 22;461(7267):1139-43. doi: 10.1038/nature08475. Nature. 2009. PMID: 19847268 Free PMC article.
-
Biochemistry. Anatomy of a fungal polyketide synthase.Science. 2008 Apr 11;320(5873):186-7. doi: 10.1126/science.1157677. Science. 2008. PMID: 18403699 No abstract available.
-
Starter unit flexibility for engineered product synthesis by the nonreducing polyketide synthase PksA.ACS Chem Biol. 2015 Jun 19;10(6):1443-9. doi: 10.1021/acschembio.5b00005. Epub 2015 Mar 10. ACS Chem Biol. 2015. PMID: 25714897 Free PMC article.
-
Probing the structure and function of acyl carrier proteins to unlock the strategic redesign of type II polyketide biosynthetic pathways.J Biol Chem. 2021 Jan-Jun;296:100328. doi: 10.1016/j.jbc.2021.100328. Epub 2021 Jan 23. J Biol Chem. 2021. PMID: 33493513 Free PMC article. Review.
Cited by
-
Characterization of the biosynthetic genes for 10,11-dehydrocurvularin, a heat shock response-modulating anticancer fungal polyketide from Aspergillus terreus.Appl Environ Microbiol. 2013 Mar;79(6):2038-47. doi: 10.1128/AEM.03334-12. Epub 2013 Jan 18. Appl Environ Microbiol. 2013. PMID: 23335766 Free PMC article.
-
Demonstration of starter unit interprotein transfer from a fatty acid synthase to a multidomain, nonreducing polyketide synthase.Chembiochem. 2012 Sep 3;13(13):1880-4. doi: 10.1002/cbic.201200267. Epub 2012 Jul 13. Chembiochem. 2012. PMID: 22807303 Free PMC article.
-
The architectures of iterative type I PKS and FAS.Nat Prod Rep. 2018 Oct 17;35(10):1046-1069. doi: 10.1039/c8np00039e. Nat Prod Rep. 2018. PMID: 30137093 Free PMC article. Review.
-
Characterization of NRPS and PKS genes involved in the biosynthesis of SMs in Alternaria dauci including the phytotoxic polyketide aldaulactone.Sci Rep. 2022 May 17;12(1):8155. doi: 10.1038/s41598-022-11896-0. Sci Rep. 2022. PMID: 35581239 Free PMC article.
-
Structural analysis of protein-protein interactions in type I polyketide synthases.Crit Rev Biochem Mol Biol. 2013 Mar-Apr;48(2):98-122. doi: 10.3109/10409238.2012.745476. Epub 2012 Dec 19. Crit Rev Biochem Mol Biol. 2013. PMID: 23249187 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

