Efficient serum-free derivation of oligodendrocyte precursors from neural stem cell-enriched cultures
- PMID: 18403757
- PMCID: PMC4772902
- DOI: 10.1634/stemcells.2007-0205
Efficient serum-free derivation of oligodendrocyte precursors from neural stem cell-enriched cultures
Abstract
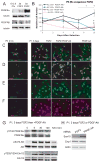
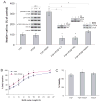
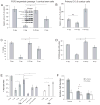
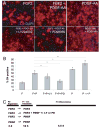
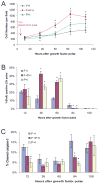
Oligodendrocytes derived in the laboratory from stem cells have been proposed as a treatment for acute and chronic injury to the central nervous system. Platelet-derived growth factor (PDGF) receptor alpha (PDGFRalpha) signaling is known to regulate oligodendrocyte precursor cell numbers both during development and adulthood. Here, we analyze the effects of PDGFRalpha signaling on central nervous system (CNS) stem cell-enriched cultures. We find that AC133 selection for CNS progenitors acutely isolated from the fetal cortex enriches for PDGF-AA-responsive cells. PDGF-AA treatment of fibroblast growth factor 2-expanded CNS stem cell-enriched cultures increases nestin(+) cell number, viability, proliferation, and glycolytic rate. We show that a brief exposure to PDGF-AA rapidly and efficiently permits the derivation of O4(+) oligodendrocyte-lineage cells from CNS stem cell-enriched cultures. The derivation of oligodendrocyte-lineage cells demonstrated here may support the effective use of stem cells in understanding fate choice mechanisms and the development of new therapies targeting this cell type.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures
References
-
- McKay R. Stem cells in the central nervous system. Science. 1997 Apr 4;276(5309):66–71. - PubMed
-
- Gage FH. Mammalian neural stem cells. Science. 2000 Feb 25;287(5457):1433–1438. - PubMed
-
- Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996 Sep;59(1):89–102. - PubMed
-
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001 Dec;19(12):1129–1133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

