BIM and other BCL-2 family proteins exhibit cross-species conservation of function between zebrafish and mammals
- PMID: 18404156
- PMCID: PMC3212414
- DOI: 10.1038/cdd.2008.42
BIM and other BCL-2 family proteins exhibit cross-species conservation of function between zebrafish and mammals
Abstract
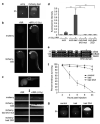
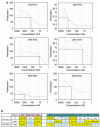
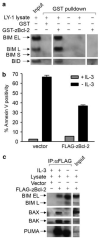
Here we investigate the function of zebrafish Bcl-2 family proteins and demonstrate important conservation of function across zebrafish and mammalian systems. We have isolated a zebrafish ortholog of mammalian BIM and show that it is the most toxic of the zebrafish BH3-only genes examined, sharing this characteristic with the mammalian BIM gene. The zebrafish bad gene shows a complete lack of embryonic lethality, but like mammalian BAD, its pro-apoptotic activity is regulated through phosphorylation of critical serines. We also found that the pattern of mitochondrial dysfunction observed by zebrafish BH3 domain peptides in a mammalian cytochrome c release assay recapitulates the pattern of embryonic lethality induced by the respective mRNA injections in vivo. In contrast to zebrafish Bim, Bid exhibited only weak binding to zebrafish Bcl-2 and moderate-to-weak overall lethality in zebrafish embryos and isolated mitochondria. Given that zebrafish Bcl-2 binds strongly to mammalian BID and BIM peptides and proteins, the protein identified as the zebrafish Bid ortholog has different properties than mammalian BID. Overall, our results demonstrate the high degree of functional conservation between zebrafish and mammalian Bcl-2 family proteins, thus validating the zebrafish as a model system to further dissect the molecular mechanisms that regulate apoptosis in future forward genetic and chemical modifier screens.
Figures



Similar articles
-
Molecular basis for Bcl-2 homology 3 domain recognition in the Bcl-2 protein family: identification of conserved hot spot interactions.J Biol Chem. 2009 Jun 26;284(26):17499-511. doi: 10.1074/jbc.M805542200. Epub 2009 Mar 17. J Biol Chem. 2009. PMID: 19293158 Free PMC article.
-
Anti-apoptotic Bcl-XL protein in complex with BH3 peptides of pro-apoptotic Bak, Bad, and Bim proteins: comparative molecular dynamics simulations.Proteins. 2008 Nov 1;73(2):492-514. doi: 10.1002/prot.22075. Proteins. 2008. PMID: 18452209
-
The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner.J Biol Chem. 2011 Mar 18;286(11):9382-92. doi: 10.1074/jbc.M110.203638. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148306 Free PMC article.
-
Role of Bim and other Bcl-2 family members in autoimmune and degenerative diseases.Curr Dir Autoimmun. 2006;9:74-94. doi: 10.1159/000090773. Curr Dir Autoimmun. 2006. PMID: 16394656 Review.
-
Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels.Cell Cycle. 2007 Sep 15;6(18):2236-40. doi: 10.4161/cc.6.18.4728. Epub 2007 Jul 10. Cell Cycle. 2007. PMID: 17881896 Review.
Cited by
-
Bax, Bcl2, and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line.J Assoc Res Otolaryngol. 2013 Oct;14(5):645-59. doi: 10.1007/s10162-013-0404-1. Epub 2013 Jul 3. J Assoc Res Otolaryngol. 2013. PMID: 23821348 Free PMC article.
-
Differential Apoptotic and Mitogenic Effects of Lectins in Zebrafish.Front Endocrinol (Lausanne). 2019 Jun 5;10:356. doi: 10.3389/fendo.2019.00356. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31231312 Free PMC article.
-
Spliceosomal components protect embryonic neurons from R-loop-mediated DNA damage and apoptosis.Dis Model Mech. 2018 Feb 26;11(2):dmm031583. doi: 10.1242/dmm.031583. Dis Model Mech. 2018. PMID: 29419415 Free PMC article.
-
Repression of BIM mediates survival signaling by MYC and AKT in high-risk T-cell acute lymphoblastic leukemia.Leukemia. 2014 Sep;28(9):1819-27. doi: 10.1038/leu.2014.78. Epub 2014 Feb 20. Leukemia. 2014. PMID: 24552990 Free PMC article.
-
Evaluation of Epigenetic and Radiomodifying Effects during Radiotherapy Treatments in Zebrafish.Int J Mol Sci. 2021 Aug 22;22(16):9053. doi: 10.3390/ijms22169053. Int J Mol Sci. 2021. PMID: 34445758 Free PMC article. Review.
References
-
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. - PubMed
-
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. - PubMed
-
- Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. - PubMed
-
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. - PubMed
-
- Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

