A generalized allosteric mechanism for cis-regulated cyclic nucleotide binding domains
- PMID: 18404204
- PMCID: PMC2275311
- DOI: 10.1371/journal.pcbi.1000056
A generalized allosteric mechanism for cis-regulated cyclic nucleotide binding domains
Erratum in
- PLoS Comput Biol. 2009 Jul;5(7). doi: 10.1371/annotation/43fd4ca3-0996-462c-ad89-395c369cbaa2 doi: 10.1371/annotation/43fd4ca3-0996-462c-ad89-395c369cbaa2
Abstract
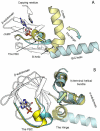
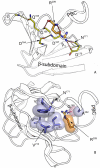
Cyclic nucleotides (cAMP and cGMP) regulate multiple intracellular processes and are thus of a great general interest for molecular and structural biologists. To study the allosteric mechanism of different cyclic nucleotide binding (CNB) domains, we compared cAMP-bound and cAMP-free structures (PKA, Epac, and two ionic channels) using a new bioinformatics method: local spatial pattern alignment. Our analysis highlights four major conserved structural motifs: 1) the phosphate binding cassette (PBC), which binds the cAMP ribose-phosphate, 2) the "hinge," a flexible helix, which contacts the PBC, 3) the beta(2,3) loop, which provides precise positioning of an invariant arginine from the PBC, and 4) a conserved structural element consisting of an N-terminal helix, an eight residue loop and the A-helix (N3A-motif). The PBC and the hinge were included in the previously reported allosteric model, whereas the definition of the beta(2,3) loop and the N3A-motif as conserved elements is novel. The N3A-motif is found in all cis-regulated CNB domains, and we present a model for an allosteric mechanism in these domains. Catabolite gene activator protein (CAP) represents a trans-regulated CNB domain family: it does not contain the N3A-motif, and its long range allosteric interactions are substantially different from the cis-regulated CNB domains.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
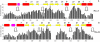

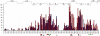


References
-
- Beavo JA, Brunton LL. Cyclic nucleotide research - still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. - PubMed
-
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. - PubMed
-
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. - PubMed
-
- Kopperud R, Krakstad C, Selheim F, Doskeland SO. cAMP effector mechanisms. Novel twists for an ‘old’ signaling system. FEBS Lett. 2003;546:121–126. - PubMed
-
- Newton RP, Smith CJ. Cyclic nucleotides. Phytochemistry. 2004;65:2423–2437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

