Tentative T cells: memory cells are quick to respond, but slow to divide
- PMID: 18404208
- PMCID: PMC2275797
- DOI: 10.1371/journal.ppat.1000041
Tentative T cells: memory cells are quick to respond, but slow to divide
Abstract
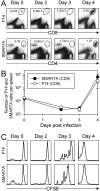
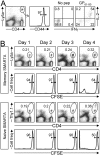
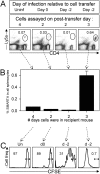
T cell memory is a cornerstone of protective immunity, and is the key element in successful vaccination. Upon encountering the relevant pathogen, memory T cells are thought to initiate cell division much more rapidly than their naïve counterparts, and this is thought to confer a significant biological advantage upon an immune host. Here, we use traceable TCR-transgenic T cells to evaluate this proposed characteristic in CD4+ and CD8+ memory T cells. We find that, even in the presence of abundant antigen that was sufficient to induce in vivo IFNgamma production by memory T cells, both memory and naïve T cells show an extended, and indistinguishable, delay in the onset of proliferation. Although memory cells can detect, and respond to, virus infection within a few hours, their proliferation did not begin until approximately 3 days after infection, and occurred simultaneously in all anatomical compartments. Thereafter, cell division was extraordinarily rapid for both naïve and memory cells, with the latter showing a somewhat accelerated accumulation. We propose that, by permitting memory T cells to rapidly exert their effector functions while delaying the onset of their proliferation, evolution has provided a safeguard that balances the risk of infection against the consequences of severe T cell-mediated immunopathology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. - PubMed
-
- Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. - PubMed
-
- Slifka MK, Whitton JL. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. - PubMed
-
- Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

