Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements
- PMID: 18404215
- PMCID: PMC2274953
- DOI: 10.1371/journal.pgen.1000050
Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements
Abstract
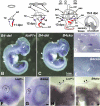
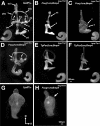
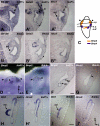
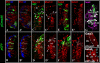
Angular head movements in vertebrates are detected by the three semicircular canals of the inner ear and their associated sensory tissues, the cristae. Bone morphogenetic protein 4 (Bmp4), a member of the Transforming growth factor family (TGF-beta), is conservatively expressed in the developing cristae in several species, including zebrafish, frog, chicken, and mouse. Using mouse models in which Bmp4 is conditionally deleted within the inner ear, as well as chicken models in which Bmp signaling is knocked down specifically in the cristae, we show that Bmp4 is essential for the formation of all three cristae and their associated canals. Our results indicate that Bmp4 does not mediate the formation of sensory hair and supporting cells within the cristae by directly regulating genes required for prosensory development in the inner ear such as Serrate1 (Jagged1 in mouse), Fgf10, and Sox2. Instead, Bmp4 most likely mediates crista formation by regulating Lmo4 and Msx1 in the sensory region and Gata3, p75Ngfr, and Lmo4 in the non-sensory region of the crista, the septum cruciatum. In the canals, Bmp2 and Dlx5 are regulated by Bmp4, either directly or indirectly. Mechanisms involved in the formation of sensory organs of the vertebrate inner ear are thought to be analogous to those regulating sensory bristle formation in Drosophila. Our results suggest that, in comparison to sensory bristles, crista formation within the inner ear requires an additional step of sensory and non-sensory fate specification.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Chang W, Cole LK, Cantos R, Wu DK, editors. Molecular Genetics of vestibular organ development. New York: Springer-Verlag; 2003. pp. 11–56.
-
- Retzius G. Das Gehororgan der Wirbelthiere: Morphologisch-histologische Studien. Stockholm:in Commission bei Samson & Wallin 1881
-
- Wersall J, Bagger-Sjoback D. Morphology of the Vestibular Sense Organ. In: Autrum H, Jung R, Loenstein WR, Mackay DM, editors. Handbook of Sensory Physiology, Vestibular System Part I. New York: Springer-Verlag; 1974. pp. 124–170.
-
- Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol. 2005;93:267–280. - PubMed
-
- Landolt JP, Young ER, Correia MJ. Vestibular ampullary structures in the pigeon: a scanning electron microscope overview. Anat Rec. 1972;174:311–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

