GPR80/99, proposed to be the P2Y(15) receptor activated by adenosine and AMP, is not a P2Y receptor
- PMID: 18404402
- PMCID: PMC2096561
- DOI: 10.1007/s11302-004-5069-0
GPR80/99, proposed to be the P2Y(15) receptor activated by adenosine and AMP, is not a P2Y receptor
Abstract
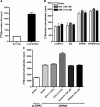
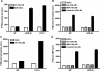
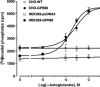
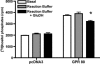
The orphan receptor GPR80 (also called GPR99) was recently reported to be the P2Y(15) receptor activated by AMP and adenosine and coupled to increases in cyclic AMP accumulation and intracellular Ca(2+) mobilization (Inbe et al. J Biol Chem 2004; 279: 19790-9). However, the cell line (HEK293) used to carry out those studies endogenously expresses A(2A) and A(2B) adenosine receptors as well as multiple P2Y receptors, which complicates the analysis of a potential P2Y receptor. To determine unambiguously whether GPR80 is a P2Y receptor subtype, HA-tagged GPR80 was either stably expressed in CHO cells or transiently expressed in COS-7 and HEK293 cells, and cell surface expression was verified by radioimmunoassay (RIA). COS-7 cells overexpressing GPR80 showed a consistent twofold increase in basal inositol phosphate accumulation. However, neither adenosine nor AMP was capable of promoting accumulation of either cyclic AMP or inositol phosphates in any of the three GPR80-expressing cells. A recent paper (He et al. Nature 2004; 429: 188-93) reported that GPR80 is a Gq-coupled receptor activated by the citric acid cycle intermediate, alpha-ketoglutarate. Consistent with this report, alpha-ketoglutarate promoted inositol phosphate accumulation in CHO and HEK293 cells expressing GPR80, and pretreatment of GPR80-expressing COS-7 cells with glutamate dehydrogenase, which converts alpha-ketoglutarate to glutamate, decreased basal levels of inositol phosphates. Taken together, these data demonstrate that GPR80 is not activated by adenosine, AMP or other nucleotides, but instead is activated by alpha-ketoglutarate. Therefore, GPR80 is not a new member of the P2Y receptor family.
Figures

References
-
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

