P1 receptors and cytokine secretion
- PMID: 18404415
- PMCID: PMC2096764
- DOI: 10.1007/s11302-006-9033-z
P1 receptors and cytokine secretion
Abstract
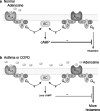
Evidence has accumulated in the last three decades to suggest tissue protection and regeneration by adenosine in multiple different cell types. Adenosine produced in hypoxic or inflamed environments reduces tissue injury and promotes repair by receptor-mediated mechanisms. Among other actions, regulation of cytokine production and secretion by immune cells, astrocytes and microglia (the brain immunocytes) has emerged as a main mechanism at the basis of adenosine effects in diseases characterized by a marked inflammatory component. Many recent studies have highlighted that signalling through A(1) and A(2A) adenosine receptors can powerfully prevent the release of pro-inflammatory cytokines, thus inhibiting inflammation and reperfusion injury. However, the activation of adenosine receptors is not invariably protective of tissues, as signalling through the A(2B) adenosine receptor has been linked to pro-inflammatory actions which are, at least in part, mediated by increased release of pro-inflammatory cytokines from epithelial cells, astrocytes and fibroblasts. Here, we discuss the multiple actions of P1 receptors on cytokine secretion, by analyzing, in particular, the role of the various adenosine receptor subtypes, the complex reciprocal interplay between the adenosine and the cytokine systems, their pathophysiological significance and the potential of adenosine receptor ligands as new anti-inflammatory agents.
Figures


References
-
- Hirschhorn R, Grossman J, Weissmann G. Effect of cyclic 3′5′-adenosine monophosphate and theophylline on lymphocyte transformation. Proc Soc Exp Biol Med. 1970;133:1361–1365. - PubMed
-
- Henney CS, Lichtenstein LM. The role of cyclic AMP in the cytolytic activity of lymphocytes. J Immunol. 1971;107:610–612. - PubMed
LinkOut - more resources
Full Text Sources

