Extracellular NAD and ATP: Partners in immune cell modulation
- PMID: 18404420
- PMCID: PMC2096762
- DOI: 10.1007/s11302-006-9038-7
Extracellular NAD and ATP: Partners in immune cell modulation
Abstract
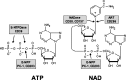
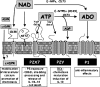
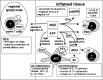
Extracellular NAD and ATP exert multiple, partially overlapping effects on immune cells. Catabolism of both nucleotides by extracellular enzymes keeps extracellular concentrations low under steady-state conditions and generates metabolites that are themselves signal transducers. ATP and its metabolites signal through purinergic P2 and P1 receptors, whereas extracellular NAD exerts its effects by serving as a substrate for ADP-ribosyltransferases (ARTs) and NAD glycohydrolases/ADPR cyclases like CD38 and CD157. Both nucleotides activate the P2X7 purinoceptor, although by different mechanisms and with different characteristics. While ATP activates P2X7 directly as a soluble ligand, activation via NAD occurs by ART-dependent ADP-ribosylation of cell surface proteins, providing an immobilised ligand. P2X7 activation by either route leads to phosphatidylserine exposure, shedding of CD62L, and ultimately to cell death. Activation by ATP requires high micromolar concentrations of nucleotide and is readily reversible, whereas NAD-dependent stimulation begins at low micromolar concentrations and is more stable. Under conditions of cell stress or inflammation, ATP and NAD are released into the extracellular space from intracellular stores by lytic and non-lytic mechanisms, and may serve as "danger signals" to alert the immune response to tissue damage. Since ART expression is limited to naïve/resting T cells, P2X7-mediated NAD-induced cell death (NICD) specifically targets this cell population. In inflamed tissue, NICD may inhibit bystander activation of unprimed T cells, reducing the risk of autoimmunity. In draining lymph nodes, NICD may eliminate regulatory T cells or provide space for the preferential expansion of primed cells, and thus help to augment an immune response.
Figures
References
-
- Herceg Z, Wang ZQ. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001;477:97–110. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

