Guanosine effect on cholesterol efflux and apolipoprotein E expression in astrocytes
- PMID: 18404467
- PMCID: PMC2096658
- DOI: 10.1007/s11302-006-9011-5
Guanosine effect on cholesterol efflux and apolipoprotein E expression in astrocytes
Abstract
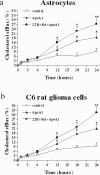
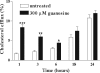
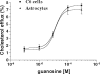
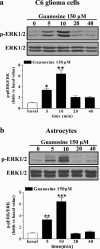
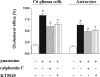
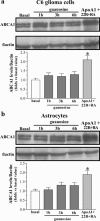
The main source of cholesterol in the central nervous system (CNS) is represented by glial cells, mainly astrocytes, which also synthesise and secrete apolipoproteins, in particular apolipoprotein E (ApoE), the major apolipoprotein in the brain, thus generating cholesterol-rich high density lipoproteins (HDLs). This cholesterol trafficking, even though still poorly known, is considered to play a key role in different aspects of neuronal plasticity and in the stabilisation of synaptic transmission. Moreover, cell cholesterol depletion has recently been linked to a reduction in amyloid beta formation. Here we demonstrate that guanosine, which we previously reported to exert several neuroprotective effects, was able to increase cholesterol efflux from astrocytes and C6 rat glioma cells in the absence of exogenously added acceptors. In this effect the phosphoinositide 3 kinase/extracellular signal-regulated kinase 1/2 (PI3K/ERK1/2) pathway seems to play a pivotal role. Guanosine was also able to increase the expression of ApoE in astrocytes, whereas it did not modify the levels of ATP-binding cassette protein A1 (ABCA1), considered the main cholesterol transporter in the CNS. Given the emerging role of cholesterol balance in neuronal repair, these effects provide evidence for a role of guanosine as a potential pharmacological tool in the modulation of cholesterol homeostasis in the brain.
Figures




References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11481254', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11481254/'}]}
- Koudinov AR, Koudinova NV (2001) Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J 15:1858′860 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.mcn.2005.02.006', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.mcn.2005.02.006'}, {'type': 'PubMed', 'value': '15911344', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15911344/'}]}
- Goritz C, Mauch DH, Pfrieger FW (2005) Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci 29:190′01 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.M308798200', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.m308798200'}, {'type': 'PubMed', 'value': '14660672', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/14660672/'}]}
- Taverna E, Saba E, Rowe J et al (2004) Role of lipid microdomains in P/Q-type calcium channel (Cav2.1) clustering and function in presynaptic membranes. J Biol Chem 279:5127′134 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11571339', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11571339/'}]}
- Simons M, Keller P, Dichgans J et al (2001) Cholesterol and Alzheimer’s disease: is there a link? Neurology 57:1089′093 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1073/pnas.95.11.6460', 'is_inner': False, 'url': 'https://doi.org/10.1073/pnas.95.11.6460'}, {'type': 'PMC', 'value': 'PMC27798', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC27798/'}, {'type': 'PubMed', 'value': '9600988', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9600988/'}]}
- Simons M, Keller P, De Strooper B et al (1998) Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 95:6460′464 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

