Modulation of purinergic signaling by NPP-type ectophosphodiesterases
- PMID: 18404476
- PMCID: PMC2254485
- DOI: 10.1007/s11302-005-5303-4
Modulation of purinergic signaling by NPP-type ectophosphodiesterases
Abstract
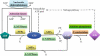
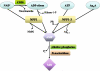
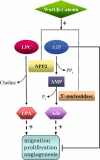
Extracellular nucleotides can elicit a wide array of cellular responses by binding to specific purinergic receptors. The level of ectonucleotides is dynamically controlled by their release from cells, synthesis by ectonucleoside diphosphokinases and ectoadenylate kinases, and hydrolysis by ectonucleotidases. One of the four structurally unrelated families of ectonucleotidases is represented by the NPP-type ectophosphodiesterases. Three of the seven members of the NPP family, namely NPP1-3, are known to hydrolyze nucleotides. The enzymatic action of NPP1-3 (in)directly results in the termination of nucleotide signaling, the salvage of nucleotides and/or the generation of new messengers like ADP, adenosine or pyrophosphate. NPP2 is unique in that it hydrolyzes both nucleotides and lysophospholipids and, thereby, generates products that could synergistically promote cell motility. We review here the enzymatic properties of NPPs and analyze current evidence that links their nucleotide-hydrolyzing capability to epithelial and neural functions, the immune response and cell motility.
Figures
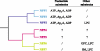

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

