Physiologic and pathologic functions of the NPP nucleotide pyrophosphatase/phosphodiesterase family focusing on NPP1 in calcification
- PMID: 18404477
- PMCID: PMC2254483
- DOI: 10.1007/s11302-005-5304-3
Physiologic and pathologic functions of the NPP nucleotide pyrophosphatase/phosphodiesterase family focusing on NPP1 in calcification
Abstract
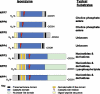
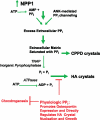
The catabolism of ATP and other nucleotides participates partly in the important function of nucleotide salvage by activated cells and also in removal or de novo generation of compounds including ATP, ADP, and adenosine that stimulate purinergic signaling. Seven nucleotide pyrophosphatase/phosphodiesterase NPP family members have been identified to date. These isoenzymes, related by up conservation of catalytic domains and certain other modular domains, exert generally non-redundant functions via distinctions in substrates and/or cellular localization. But they share the capacity to hydrolyze phosphodiester or pyrophosphate bonds, though generally acting on distinct substrates that include nucleoside triphosphates, lysophospholipids and choline phosphate esters. PP(i) generation from nucleoside triphosphates, catalyzed by NPP1 in tissues including cartilage, bone, and artery media smooth muscle cells, supports normal tissue extracellular PP(i) levels. Balance in PP(i) generation relative to PP(i) degradation by pyrophosphatases holds extracellular PP(i) levels in check. Moreover, physiologic levels of extracellular PP(i) suppress hydroxyapatite crystal growth, but concurrently providing a reservoir for generation of pro-mineralizing P(i). Extracellular PP(i) levels must be supported by cells in mineralization-competent tissues to prevent pathologic calcification. This support mechanism becomes dysregulated in aging cartilage, where extracellular PP(i) excess, mediated in part by upregulated NPP1 expression stimulates calcification. PP(i) generated by NPP1modulates not only hydroxyapatite crystal growth but also chondrogenesis and expression of the mineralization regulator osteopontin. This review pays particular attention to the role of NPP1-catalyzed PP(i) generation in the pathogenesis of certain disorders associated with pathologic calcification.
Figures
References
-
- Deterre P, Gelman L, Gary-Gouy H et al. Coordinated regulation in human T cells of nucleotide-hydrolyzing ecto-enzymatic activities, including CD38 and PC-1. Possible role in the recycling of nicotinamide adenine dinucleotide metabolites. J Immunol 1996; 157: 1381–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

