CD39, NTPDase 1, is attached to the plasma membrane by two transmembrane domains. Why?
- PMID: 18404478
- PMCID: PMC2254477
- DOI: 10.1007/s11302-005-5907-8
CD39, NTPDase 1, is attached to the plasma membrane by two transmembrane domains. Why?
Abstract
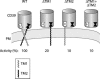
Since the identification of CD39 and other members of the e-NTPDase (ecto-nucleoside triphosphate diphosphohydrolase) family as the primary enzymes responsible for cell surface nucleotide hydrolysis, one of their most intriguing features has been their unusual topology. The active site lies in the large extracellular region, but instead of being anchored in the membrane by a single transmembrane domain or lipid link like other ectoenzymes, CD39 has two transmembrane domains, one at each end. In this review we discuss evidence that the structure and dynamics of the transmembrane helices are intricately connected to enzymatic function. Removal of either or both transmembrane domains or disruption of their native state by detergent solubilization reduces activity by 90%, indicating that native function requires both transmembrane domains to be present and in the membrane. Enzymatic and mutational analysis of the native and truncated forms has shown that the active site can exist in distinct functional states characterized by different total activities, substrate specificities, hydrolysis mechanisms, and intermediate ADP release during ATP hydrolysis, depending on the state of the transmembrane domains. Disulfide crosslinking of cysteines introduced within the transmembrane helices revealed that they interact within and between molecules, in particular near the extracellular domain, and that activity depends on their organization. Both helices exhibit a high degree of rotational mobility, and the ability to undergo dynamic motions is required for activity and regulated by substrate binding. Recent reports suggest that membrane composition can regulate NTPDase activity. We propose that mechanical bilayer properties, potentially elasticity, might regulate CD39 by altering the balance between stability and mobility of its transmembrane domains.
Figures
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '13050757', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/13050757/'}]}
- Rothstein A, Meier RC, Scharff TG. Relationship of cell surface to metabolism: IX. Digestion of phosphorylated compounds by enzymes located on the surface of intestinal cells. Am J Phys 1953; 173: 41–6. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/ddr.430320303', 'is_inner': False, 'url': 'https://doi.org/10.1002/ddr.430320303'}]}
- Ziganshin AU, Hoyle CHV, Burnstock G. Ecto-enzymes and metabolism of extracellular ATP. Drug Dev Res 1994; 32: 134–46.
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1006/bbrc.1996.0162', 'is_inner': False, 'url': 'https://doi.org/10.1006/bbrc.1996.0162'}, {'type': 'PubMed', 'value': '8579614', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8579614/'}]}
- Handa M and Guidotti G. Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum). Biochem Biophys Res Commun 1996; 218: 916–23. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.273.18.11392', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.273.18.11392'}, {'type': 'PubMed', 'value': '9556635', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9556635/'}]}
- Wang TF, Guidotti G. Golgi localization and functional expression of human uridine diphosphatase. J Biol Chem 1998; 273: 11392–11399. - PubMed
-
- None
- Zimmermann H, Beaudoin AR, Bollen M et al. Proposed nomenclature for two novel nucleotide hydrolysing enzyme families expressed on the cell surface. In vanDuffel L, Lemmens R (eds): Ecto-ATPase and Related Nucleotidases, Maastricht: Shaker, 2000, 1–8.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

