The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance
- PMID: 18404480
- PMCID: PMC2254478
- DOI: 10.1007/s11302-006-9003-5
The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance
Abstract
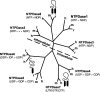
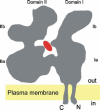
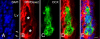
Ectonucleotidases are ectoenzymes that hydrolyze extracellular nucleotides to the respective nucleosides. Within the past decade, ectonucleotidases belonging to several enzyme families have been discovered, cloned and characterized. In this article, we specifically address the cell surface-located members of the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase/CD39) family (NTPDase1,2,3, and 8). The molecular identification of individual NTPDase subtypes, genetic engineering, mutational analyses, and the generation of subtype-specific antibodies have resulted in considerable insights into enzyme structure and function. These advances also allow definition of physiological and patho-physiological implications of NTPDases in a considerable variety of tissues. Biological actions of NTPDases are a consequence (at least in part) of the regulated phosphohydrolytic activity on extracellular nucleotides and consequent effects on P2-receptor signaling. It further appears that the spatial and temporal expression of NTPDases by various cell types within the vasculature, the nervous tissues and other tissues impacts on several patho-physiological processes. Examples include acute effects on cellular metabolism, adhesion, activation and migration with other protracted impacts upon developmental responses, inclusive of cellular proliferation, differentiation and apoptosis, as seen with atherosclerosis, degenerative neurological diseases and immune rejection of transplanted organs and cells. Future clinical applications are expected to involve the development of new therapeutic strategies for transplantation and various inflammatory cardiovascular, gastrointestinal and neurological diseases.
Figures


References
-
- Luthje J (1989) Origin, metabolism and function of extracellular adenine nucleotides in the blood. Klin Wochenschr 67:317–327 - PubMed
-
- Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304 - PubMed
-
- Lazarowski ER (2003) Molecular and biological properties of P2Y receptors. In: Schwiebert EM (ed) Extracellular Nucleotides and Nucleosides: Release, Receptors, and Physiological and Pathophysiological Effects. Academic, Amsterdam, Boston, pp 59–96
-
- Harden TK, Lazarowski ER, Boucher RC (1997) Release, metabolism and interconversion of adenine and uridine nucleotides: Implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol Sci 18:43–46 - PubMed
-
- Weisman GA, Erb L, Garrad RC et al (1998) P2Y nucleotide receptors in the immune system: Signaling by a P2Y2 receptor in U937 monocytes. Drug Dev Res 45:222–228
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

