Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1-3 in hypothalamic and pituitary cells
- PMID: 18404498
- PMCID: PMC2096527
- DOI: 10.1007/s11302-005-6208-y
Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1-3 in hypothalamic and pituitary cells
Abstract
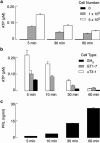
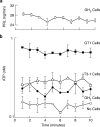
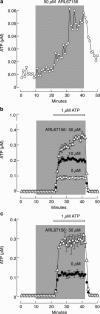
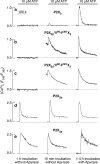
Hypothalamic and pituitary cells express G protein-coupled adenosine and P2Y receptors and cation-conducting P2X receptor-channels, suggesting that extracellular ATP and other nucleotides may function as autocrine and/or paracrine signaling factors in these cells. Consistent with this hypothesis, we show that cultured normal and immortalized pituitary and hypothalamic cells release ATP under resting conditions. RT-PCR analysis also revealed the presence of transcripts for ecto-nucleotidase eNTPDase 1-3 in these cells. These enzymes were functional as documented by degradation of endogenously released and exogenously added ATP. Blocking the activity of eNTPDases by ARL67156 led to an increase in ATP release in perifused pituitary cells and inhibition of degradation of extracellularly added ATP. Furthermore, the addition of apyrase, a soluble ecto-nucleotidase, and the expression of recombinant mouse eNTPDase-2, enhanced degradation of both endogenously released and exogenously added ATP. The released ATP by resting hypothalamic cells was sufficient to activate and desensitize high-affinity recombinant P2X receptors, whereas facilitation of ATP metabolism by the addition of apyrase protected their desensitization. These results indicate that colocalization of ATP release sites and ecto-nucleotidase activity at the plasma membrane of hypothalamic and pituitary cells provides an effective mechanism for the operation of nucleotides as extracellular signaling molecules.
Figures

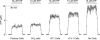

Similar articles
-
Stage-specific expression of P2Y receptors, ecto-apyrase, and ecto-5'-nucleotidase in myeloid leukocytes.Am J Physiol. 1997 Sep;273(3 Pt 1):C973-87. doi: 10.1152/ajpcell.1997.273.3.C973. Am J Physiol. 1997. PMID: 9316419
-
Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons.Br J Pharmacol. 2009 Feb;156(3):519-33. doi: 10.1111/j.1476-5381.2008.00058.x. Epub 2009 Jan 13. Br J Pharmacol. 2009. PMID: 19154428 Free PMC article.
-
Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes.J Biol Chem. 2003 Jun 27;278(26):23331-42. doi: 10.1074/jbc.M302680200. Epub 2003 Apr 8. J Biol Chem. 2003. PMID: 12684505
-
Signaling by extracellular nucleotides in anterior pituitary cells.Trends Endocrinol Metab. 2001 Jul;12(5):218-25. doi: 10.1016/s1043-2760(01)00387-3. Trends Endocrinol Metab. 2001. PMID: 11397647 Review.
-
Extracellular ATP and adenosine: The Yin and Yang in immune responses?Mol Aspects Med. 2017 Jun;55:9-19. doi: 10.1016/j.mam.2017.01.002. Epub 2017 Jan 16. Mol Aspects Med. 2017. PMID: 28093236 Review.
Cited by
-
Purinergic signaling pathways in endocrine system.Auton Neurosci. 2015 Sep;191:102-16. doi: 10.1016/j.autneu.2015.04.010. Epub 2015 Apr 25. Auton Neurosci. 2015. PMID: 25960051 Free PMC article. Review.
-
Adenine Nucleotides Control Proliferation In Vivo of Rat Retinal Progenitors by P2Y1 Receptor.Mol Neurobiol. 2017 Sep;54(7):5142-5155. doi: 10.1007/s12035-016-0059-0. Epub 2016 Aug 24. Mol Neurobiol. 2017. PMID: 27558237
-
The F0F1 ATP Synthase Complex Localizes to Membrane Rafts in Gonadotrope Cells.Mol Endocrinol. 2016 Sep;30(9):996-1011. doi: 10.1210/me.2015-1324. Epub 2016 Aug 2. Mol Endocrinol. 2016. PMID: 27482602 Free PMC article.
-
Synthesis of new class of indole acetic acid sulfonate derivatives as ectonucleotidases inhibitors.RSC Adv. 2023 Oct 10;13(42):29496-29511. doi: 10.1039/d3ra04266a. eCollection 2023 Oct 4. RSC Adv. 2023. PMID: 37822663 Free PMC article.
-
The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance.Purinergic Signal. 2006 Jun;2(2):409-30. doi: 10.1007/s11302-006-9003-5. Epub 2006 May 30. Purinergic Signal. 2006. PMID: 18404480 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s002100000309', 'is_inner': False, 'url': 'https://doi.org/10.1007/s002100000309'}, {'type': 'PubMed', 'value': '11111825', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11111825/'}]}
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch Pharmacol 2000; 362: 299–309. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '9755289', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9755289/'}]}
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998; 50: 413–492. - PubMed
-
- None
- Pearson JD. Ectonucleotidases: Measurement of activities and use of inhibitors. Methods Pharmacol 1985; 6: 83–107.
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1042/CS20030053', 'is_inner': False, 'url': 'https://doi.org/10.1042/cs20030053'}, {'type': 'PubMed', 'value': '12578557', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12578557/'}]}
- Rees DA, Scanlon MF, Ham J. Novel insights into how purines regulate pituitary cell function. Clin Sci 2003; 104: 467–471. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1677/joe.0.1770357', 'is_inner': False, 'url': 'https://doi.org/10.1677/joe.0.1770357'}, {'type': 'PubMed', 'value': '12773115', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12773115/'}]}
- Rees DA, Scanlon MF, Ham J. Adenosine signalling pathway in the pituitary gland: One ligand, multiple receptors. J Endocrinol 2003; 177: 357-4. - PubMed
LinkOut - more resources
Full Text Sources

