Tri-nucleotide receptors play a critical role in epithelial cell wound repair
- PMID: 18404512
- PMCID: PMC2096543
- DOI: 10.1007/s11302-005-8132-6
Tri-nucleotide receptors play a critical role in epithelial cell wound repair
Abstract
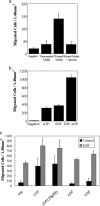
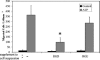
The cornea plays a major role in the refraction of light to the retina. Therefore, the integrity and transparency of the corneal epithelium are critical to vision. Following injury, a combination of rapid signal transduction events and long-term cell migration are essential for wound closure. We have demonstrated previously that injury resulted in the release of nucleotides that induce the propagation of a Ca(2+) wave to neighboring cells. This suggests that nucleotides and their receptors are critical components of wound healing. Epidermal growth factor (EGF) and integrins also have been shown to play a role in injury. In this study, we demonstrate that pretreatment of cells with ATP and UTP inhibited the immediate wound response, while BzATP, ADP, and UDP did not affect this response. Tri-nucleotide pretreatment also reduced the EGF induced Ca(2+) response. Additionally, lower EC(50) concentrations of ATP and UTP triggered migration of cells that was enhanced further with EGF and was inhibited by the tripeptide, RGD. Results indicate that the desensitization induced by ATP and UTP was specific. While ADP and UDP cause a homologous desensitization of their own signal, they did not cause an inhibition of the wound response nor does BzATP. Neither Ca(2+) wave propagation nor cell migration occurred in response to beta,gamma-MeATP. Together these results lead us to hypothesize that corneal epithelial wound repair is mediated by both P2Y(2) and P2Y(4) receptors.
Figures







References
-
- Neary JT, Kang Y, Bu Y et al. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: Involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/Calcium pathway. J Neurosci 1999; 19(11): 4211–20. - PMC - PubMed
-
- Klepeis VE, Cornell-Bell A, Trinkaus-Randall V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J Cell Sci 2001; 114(23): 4185–95. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

