P2X(7) nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons
- PMID: 18404518
- PMCID: PMC2096553
- DOI: 10.1007/s11302-005-7145-5
P2X(7) nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons
Abstract
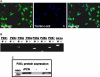
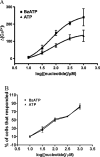
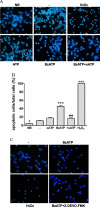
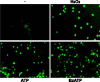
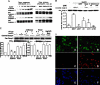
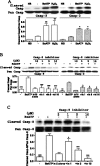
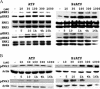
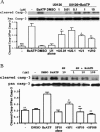
Apoptosis is a major cause of cell death in the nervous system. It plays a role in embryonic and early postnatal brain development and contributes to the pathology of neurodegenerative diseases. Here, we report that activation of the P2X(7) nucleotide receptor (P2X(7)R) in rat primary cortical neurons (rPCNs) causes biochemical (i.e., caspase activation) and morphological (i.e., nuclear condensation and DNA fragmentation) changes characteristic of apoptotic cell death. Caspase-3 activation and DNA fragmentation in rPCNs induced by the P2X(7)R agonist BzATP were inhibited by the P2X(7)R antagonist oxidized ATP (oATP) or by pre-treatment of cells with P2X(7)R antisense oligonucleotide indicating a direct involvement of the P2X(7)R in nucleotide-induced neuronal cell death. Moreover, Z-DEVD-FMK, a specific and irreversible cell permeable inhibitor of caspase-3, prevented BzATP-induced apoptosis in rPCNs. In addition, a specific caspase-8 inhibitor, Ac-IETD-CHO, significantly attenuated BzATP-induced caspase-9 and caspase-3 activation, suggesting that P2X(7)R-mediated apoptosis in rPCNs occurs primarily through an intrinsic caspase-8/9/3 activation pathway. BzATP also induced the activation of C-jun N-terminal kinase 1 (JNK1) and extracellular signal-regulated kinases (ERK1/2) in rPCNs, and pharmacological inhibition of either JNK1 or ERK1/2 significantly reduced caspase activation by BzATP. Taken together, these data indicate that extracellular nucleotides mediate neuronal apoptosis through activation of P2X(7)Rs and their downstream signaling pathways involving JNK1, ERK and caspases 8/9/3.
Figures

Similar articles
-
Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons.Invest Ophthalmol Vis Sci. 2010 Jun;51(6):3236-43. doi: 10.1167/iovs.09-4192. Epub 2010 Jan 13. Invest Ophthalmol Vis Sci. 2010. PMID: 20071682
-
Ceramide induces neuronal apoptosis through the caspase-9/caspase-3 pathway.Biochem Biophys Res Commun. 2002 Nov 29;299(2):201-7. doi: 10.1016/s0006-291x(02)02593-7. Biochem Biophys Res Commun. 2002. PMID: 12437970
-
FasL-triggered death of Jurkat cells requires caspase 8-induced, ATP-dependent cross-talk between Fas and the purinergic receptor P2X(7).J Cell Physiol. 2013 Feb;228(2):485-93. doi: 10.1002/jcp.24159. J Cell Physiol. 2013. PMID: 22806078
-
Activation of ERK1/2 by extracellular nucleotides in macrophages is mediated by multiple P2 receptors independently of P2X7-associated pore or channel formation.Br J Pharmacol. 2006 Feb;147(3):324-34. doi: 10.1038/sj.bjp.0706559. Br J Pharmacol. 2006. PMID: 16341234 Free PMC article.
-
P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system.Eur J Pharmacol. 2002 Jul 5;447(2-3):247-60. doi: 10.1016/s0014-2999(02)01756-9. Eur J Pharmacol. 2002. PMID: 12151016 Review.
Cited by
-
Alzheimer and Purinergic Signaling: Just a Matter of Inflammation?Cells. 2021 May 20;10(5):1267. doi: 10.3390/cells10051267. Cells. 2021. PMID: 34065393 Free PMC article. Review.
-
P2X7 Receptor is Involved in Mitochondrial Dysfunction Induced by Extracellular Alpha Synuclein in Neuroblastoma SH-SY5Y Cells.Int J Mol Sci. 2020 May 31;21(11):3959. doi: 10.3390/ijms21113959. Int J Mol Sci. 2020. PMID: 32486485 Free PMC article.
-
POM-1 inhibits P2 receptors and exhibits anti-inflammatory effects in macrophages.Purinergic Signal. 2017 Dec;13(4):611-627. doi: 10.1007/s11302-017-9588-x. Epub 2017 Oct 11. Purinergic Signal. 2017. PMID: 29022161 Free PMC article.
-
Recombinant Analogs of Sea Anemone Kunitz-Type Peptides Influence P2X7 Receptor Activity in Neuro-2a Cells.Mar Drugs. 2023 Mar 20;21(3):192. doi: 10.3390/md21030192. Mar Drugs. 2023. PMID: 36976241 Free PMC article.
-
Purinergic receptors as potential therapeutic targets in Alzheimer's disease.Neuropharmacology. 2016 May;104:169-79. doi: 10.1016/j.neuropharm.2015.10.031. Epub 2015 Oct 28. Neuropharmacology. 2016. PMID: 26519903 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/0014-2999(78)90070-5', 'is_inner': False, 'url': 'https://doi.org/10.1016/0014-2999(78)90070-5'}, {'type': 'PubMed', 'value': '658131', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/658131/'}]}
- Burnstock G, Cocks T, Kasakov L, Wong HK. Direct evidence for ATP release from non-adrenergic, non-cholinergic (“purinergic- nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol 1978; 49: 145-. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '6108568', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/6108568/'}]}
- Burnstock G. Purinergic nerves and receptors. Prog Biochem Pharmacol 1980; 16: 141-4. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/glia.10150', 'is_inner': False, 'url': 'https://doi.org/10.1002/glia.10150'}, {'type': 'PubMed', 'value': '12379903', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12379903/'}]}
- Inoue K. Microglial activation by purines and pyrimidines. Glia 2002; 40: 156-3. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC8653507', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC8653507/'}, {'type': 'PubMed', 'value': '12559763', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12559763/'}]}
- Abbracchio MP, Boeynaems J-M, Barnard EA et al. Characterization of the UDP-glucose receptor (renamed here the P2Y14 receptor) adds diversity to the P2Y receptor family. TiPS 2003; 24: 52-. - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10823099', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10823099/'}]}
- Burnstock G. P2X receptors in sensory neurones. Br J Anaesth 2000; 84: 476-8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

