Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation
- PMID: 18405887
- PMCID: PMC2519876
- DOI: 10.1016/j.carres.2008.03.025
Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation
Abstract
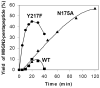
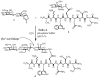
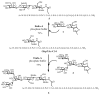
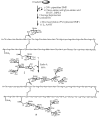
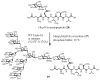
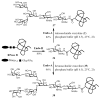
Homogeneous glycopeptides and glycoproteins are indispensable for detailed structural and functional studies of glycoproteins. It is also fundamentally important to correct glycosylation patterns for developing effective glycoprotein-based therapeutics. This review discusses a useful chemoenzymatic method that takes advantage of the endoglycosidase-catalyzed transglycosylation to attach an intact oligosaccharide to a polypeptide in a single step, without the need for any protecting groups. The exploration of sugar oxazolines (enzymatic reaction intermediates) as donor substrates has not only expanded substrate availability, but also has significantly enhanced the enzymatic transglycosylation efficiency. Moreover, the discovery of a novel mutant with glycosynthase-like activity has made it possible to synthesize homogeneous glycoproteins with full-size natural N-glycans. Recent advances in this highly convergent chemoenzymatic approach and its application for glycopeptide and glycoprotein synthesis are highlighted.
Figures

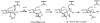






Similar articles
-
Highly efficient endoglycosidase-catalyzed synthesis of glycopeptides using oligosaccharide oxazolines as donor substrates.J Am Chem Soc. 2005 Jul 13;127(27):9692-3. doi: 10.1021/ja051715a. J Am Chem Soc. 2005. PMID: 15998066
-
Efficient transfer of sialo-oligosaccharide onto proteins by combined use of a glycosynthase-like mutant of Mucor hiemalis endoglycosidase and synthetic sialo-complex-type sugar oxazoline.Biochim Biophys Acta. 2010 Nov;1800(11):1203-9. doi: 10.1016/j.bbagen.2010.07.003. Epub 2010 Jul 18. Biochim Biophys Acta. 2010. PMID: 20647032
-
Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans.J Am Chem Soc. 2009 Feb 18;131(6):2214-23. doi: 10.1021/ja8074677. J Am Chem Soc. 2009. PMID: 19199609 Free PMC article.
-
Recent advances in glycotechnology for glycoconjugate synthesis using microbial endoglycosidases.Biotechnol Lett. 2013 Nov;35(11):1733-43. doi: 10.1007/s10529-013-1272-9. Epub 2013 Jun 26. Biotechnol Lett. 2013. PMID: 23801123 Review.
-
Glycopeptide and glycoprotein synthesis involving unprotected carbohydrate building blocks.Med Res Rev. 2005 Nov;25(6):655-78. doi: 10.1002/med.20033. Med Res Rev. 2005. PMID: 15895471 Review.
Cited by
-
Recent Progress in Chemo-Enzymatic Methods for the Synthesis of N-Glycans.Front Chem. 2020 Jun 16;8:513. doi: 10.3389/fchem.2020.00513. eCollection 2020. Front Chem. 2020. PMID: 32612979 Free PMC article. Review.
-
Convergent chemo-enzymatic synthesis of mannosylated glycopeptides; targeting of putative vaccine candidates to antigen presenting cells.Chem Sci. 2015 Aug 1;6(8):4636-4642. doi: 10.1039/c5sc00952a. Epub 2015 May 19. Chem Sci. 2015. PMID: 28717478 Free PMC article.
-
Evidence for vital role of endo-β-N-acetylglucosaminidase in the resistance of Arthrobacter protophormiae RKJ100 towards elevated concentrations of o-nitrobenzoate.Extremophiles. 2014 May;18(3):491-500. doi: 10.1007/s00792-014-0632-2. Epub 2014 Feb 23. Extremophiles. 2014. PMID: 24562786
-
Endo-F3 Glycosynthase Mutants Enable Chemoenzymatic Synthesis of Core-fucosylated Triantennary Complex Type Glycopeptides and Glycoproteins.J Biol Chem. 2016 Apr 22;291(17):9356-70. doi: 10.1074/jbc.M116.721597. Epub 2016 Mar 10. J Biol Chem. 2016. PMID: 26966183 Free PMC article.
-
Expeditious chemoenzymatic synthesis of homogeneous N-glycoproteins carrying defined oligosaccharide ligands.J Am Chem Soc. 2008 Oct 15;130(41):13790-803. doi: 10.1021/ja805044x. Epub 2008 Sep 20. J Am Chem Soc. 2008. PMID: 18803385 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

