Quantitative analysis of pathways controlling extrinsic apoptosis in single cells
- PMID: 18406323
- PMCID: PMC2858979
- DOI: 10.1016/j.molcel.2008.02.012
Quantitative analysis of pathways controlling extrinsic apoptosis in single cells
Abstract
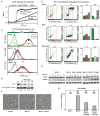
Apoptosis in response to TRAIL or TNF requires the activation of initiator caspases, which then activate the effector caspases that dismantle cells and cause death. However, little is known about the dynamics and regulatory logic linking initiators and effectors. Using a combination of live-cell reporters, flow cytometry, and immunoblotting, we find that initiator caspases are active during the long and variable delay that precedes mitochondrial outer membrane permeabilization (MOMP) and effector caspase activation. When combined with a mathematical model of core apoptosis pathways, experimental perturbation of regulatory links between initiator and effector caspases reveals that XIAP and proteasome-dependent degradation of effector caspases are important in restraining activity during the pre-MOMP delay. We identify conditions in which restraint is impaired, creating a physiologically indeterminate state of partial cell death with the potential to generate genomic instability. Together, these findings provide a quantitative picture of caspase regulatory networks and their failure modes.
Figures






References
-
- Betti CJ, Villalobos MJ, Jiang Q, Cline E, Diaz MO, Loredo G, Vaughan AT. Cleavage of the MLL gene by activators of apoptosis is independent of topoisomerase II activity. Leukemia. 2005;19:2289–2295. - PubMed
-
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. - PubMed
-
- Bratton SB, Cohen GM. Death receptors leave a caspase footprint that Smacs of XIAP. Cell Death Differ. 2003;10:4–6. - PubMed
-
- Chen L, Smith L, Wang Z, Smith JB. Preservation of caspase-3 subunits from degradation contributes to apoptosis evoked by lactacystin: any single lysine or lysine pair of the small subunit is sufficient for ubiquitination. Mol Pharmacol. 2003;64:334–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

