Function, structure and regulation of the vacuolar (H+)-ATPases
- PMID: 18406336
- PMCID: PMC2543942
- DOI: 10.1016/j.abb.2008.03.025
Function, structure and regulation of the vacuolar (H+)-ATPases
Abstract
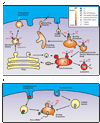
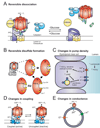
The vacuolar ATPases (or V-ATPases) are ATP-driven proton pumps that function to both acidify intracellular compartments and to transport protons across the plasma membrane. Intracellular V-ATPases function in such normal cellular processes as receptor-mediated endocytosis, intracellular membrane traffic, prohormone processing, protein degradation and neurotransmitter uptake, as well as in disease processes, including infection by influenza and other viruses and killing of cells by anthrax and diphtheria toxin. Plasma membrane V-ATPases are important in such physiological processes as urinary acidification, bone resorption and sperm maturation as well as in human diseases, including osteopetrosis, renal tubular acidosis and tumor metastasis. V-ATPases are large multi-subunit complexes composed of a peripheral domain (V(1)) responsible for hydrolysis of ATP and an integral domain (V(0)) that carries out proton transport. Proton transport is coupled to ATP hydrolysis by a rotary mechanism. V-ATPase activity is regulated in vivo using a number of mechanisms, including reversible dissociation of the V(1) and V(0) domains, changes in coupling efficiency of proton transport and ATP hydrolysis and changes in pump density through reversible fusion of V-ATPase containing vesicles. V-ATPases are emerging as potential drug targets in treating a number of human diseases including osteoporosis and cancer.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

