High titers of mucosal and systemic anti-PrP antibodies abrogate oral prion infection in mucosal-vaccinated mice
- PMID: 18407424
- PMCID: PMC2474749
- DOI: 10.1016/j.neuroscience.2008.02.051
High titers of mucosal and systemic anti-PrP antibodies abrogate oral prion infection in mucosal-vaccinated mice
Abstract
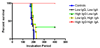
Significant outbreaks of prion disease linked to oral exposure of the prion agent have occurred in animal and human populations. These disorders are associated with a conformational change of a normal protein, PrP(C) (C for cellular), to a toxic and infectious form, PrP(Sc) (Sc for scrapie). None of the prionoses currently have an effective treatment. Some forms of prion disease are thought to be spread by oral ingestion of PrP(Sc), such as chronic wasting disease and variant Creutzfeldt-Jakob disease. Attempts to obtain an active immunization in wild-type animals have been hampered by auto-tolerance to PrP and potential toxicity. Previously, we demonstrated that it is possible to overcome tolerance and obtain a specific anti-PrP antibody response by oral inoculation of the PrP protein expressed in an attenuated Salmonella vector. This past study showed that 30% of vaccinated animals were free of disease more than 350 days post-challenge. In the current study we have both optimized the vaccination protocol and divided the vaccinated mice into low and high immune responder groups prior to oral challenge with PrP(Sc) scrapie strain 139A. These methodological refinements led to a significantly improved therapeutic response. 100% of mice with a high mucosal anti-PrP titer immunoglobulin (Ig) A and a high systemic IgG titer, prior to challenge, remained without symptoms of PrP infection at 400 days (log-rank test P<0.0001 versus sham controls). The brains from these surviving clinically asymptomatic mice were free of PrP(Sc) infection by Western blot and histological examination. These promising findings suggest that effective mucosal vaccination is a feasible and useful method for overcoming tolerance to PrP and preventing prion infection via an oral route.
Figures



Similar articles
-
Mucosal vaccination delays or prevents prion infection via an oral route.Neuroscience. 2005;133(2):413-21. doi: 10.1016/j.neuroscience.2005.02.031. Neuroscience. 2005. PMID: 15878645
-
Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease.Vaccine. 2015 Jan 29;33(5):726-33. doi: 10.1016/j.vaccine.2014.11.035. Epub 2014 Dec 21. Vaccine. 2015. PMID: 25539804 Free PMC article.
-
Could immunomodulation be used to prevent prion diseases?Expert Rev Anti Infect Ther. 2012 Mar;10(3):307-17. doi: 10.1586/eri.11.177. Expert Rev Anti Infect Ther. 2012. PMID: 22397565 Free PMC article.
-
Immunomodulation for prion and prion-related diseases.Expert Rev Vaccines. 2010 Dec;9(12):1441-52. doi: 10.1586/erv.10.131. Expert Rev Vaccines. 2010. PMID: 21105779 Free PMC article. Review.
-
Vaccine approaches to prevent and treat prion infection : progress and challenges.BioDrugs. 2008;22(1):45-52. doi: 10.2165/00063030-200822010-00005. BioDrugs. 2008. PMID: 18215090 Review.
Cited by
-
Prions--not your immunologist's pathogen.PLoS Pathog. 2015 Feb 19;11(2):e1004624. doi: 10.1371/journal.ppat.1004624. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25695738 Free PMC article. Review. No abstract available.
-
Oral Prion Neuroinvasion Occurs Independently of PrPC Expression in the Gut Epithelium.J Virol. 2018 Sep 12;92(19):e01010-18. doi: 10.1128/JVI.01010-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021891 Free PMC article.
-
Salmonella Immunotherapy Improves the Outcome of CHOP Chemotherapy in Non-Hodgkin Lymphoma-Bearing Mice.Front Immunol. 2018 Jan 23;9:7. doi: 10.3389/fimmu.2018.00007. eCollection 2018. Front Immunol. 2018. PMID: 29410666 Free PMC article.
-
Detection and control of prion diseases in food animals.ISRN Vet Sci. 2012 Feb 29;2012:254739. doi: 10.5402/2012/254739. Print 2012. ISRN Vet Sci. 2012. PMID: 23738120 Free PMC article.
-
Immunotherapy against Prion Disease.Pathogens. 2020 Mar 14;9(3):216. doi: 10.3390/pathogens9030216. Pathogens. 2020. PMID: 32183309 Free PMC article. Review.
References
-
- Aguzzi A, Sigurdson CJ. Antiprion immunotherapy: to suppress or to stimulate? Nat. Rev. Immunol. 2004;4:725–736. - PubMed
-
- Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC. Prions in skeletal muscles of deer with chronic wasting disease. Science. 2006;311:1117. - PubMed
-
- Arbel M, Lavie V, Solomon B. Generation of antibodies against prion protein in wild-type mice via helix 1 peptide immunization. J. Neuroimmunol. 2003;144:38–45. - PubMed
-
- Aucouturier P, Carp RI, Carnaud C, Wisniewski T. Prion diseases and the immune system. Clin. Immunol. 2000;96:79–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

