Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis
- PMID: 18407617
- PMCID: PMC3761971
- DOI: 10.1021/ac800112r
Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis
Abstract
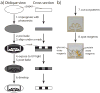
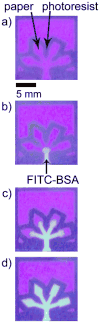
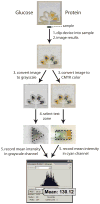
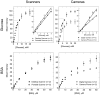
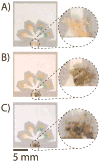
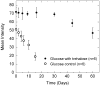
This article describes a prototype system for quantifying bioassays and for exchanging the results of the assays digitally with physicians located off-site. The system uses paper-based microfluidic devices for running multiple assays simultaneously, camera phones or portable scanners for digitizing the intensity of color associated with each colorimetric assay, and established communications infrastructure for transferring the digital information from the assay site to an off-site laboratory for analysis by a trained medical professional; the diagnosis then can be returned directly to the healthcare provider in the field. The microfluidic devices were fabricated in paper using photolithography and were functionalized with reagents for colorimetric assays. The results of the assays were quantified by comparing the intensities of the color developed in each assay with those of calibration curves. An example of this system quantified clinically relevant concentrations of glucose and protein in artificial urine. The combination of patterned paper, a portable method for obtaining digital images, and a method for exchanging results of the assays with off-site diagnosticians offers new opportunities for inexpensive monitoring of health, especially in situations that require physicians to travel to patients (e.g., in the developing world, in emergency management, and during field operations by the military) to obtain diagnostic information that might be obtained more effectively by less valuable personnel.
Figures


References
-
- Daar AS, Thorsteinsdóttir H, Martin DK, Smith AC, Nast S, Singer PA. Nat Genet. 2002;32:229–232. - PubMed
-
- Mabey D, Peeling RW, Ustianowski A, Perkins MD. Nat Rev Microbiol. 2004;2:231–240. - PubMed
-
- Braun RP, Vecchietti JL, Thomas L, Prins C, French LE, Gewirtzman AJ, Saurat JH, Salomon D. Arch Dermatol. 2005;141:254–258. - PubMed
-
- Dziadzio M, Hamdulay S, Reddy V, Boyce S, Keat A, Andrews J. Clin Rheumatol. 2007;26:979–980. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

