A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease
- PMID: 18407999
- PMCID: PMC2440599
- DOI: 10.1074/jbc.M801192200
A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease
Abstract
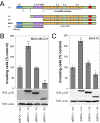
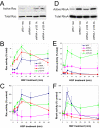
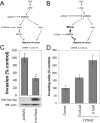
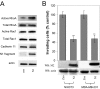
p120 catenin is a cadherin-associated protein that regulates Rho GTPases and promotes the invasiveness of E-cadherin-deficient cancer cells. Multiple p120 isoforms are expressed in cells via alternative splicing, and all of them are essential for HGF signaling to Rac1. However, only full-length p120 (isoform 1) promotes invasiveness. This selective ability of p120 isoform 1 is mediated by reduced RhoA activity, both under basal conditions and following HGF treatment. All p120 isoforms can bind RhoA in vitro, via a central RhoA binding site. However, only the cooperative binding of RhoA to the central p120 domain and to the alternatively spliced p120 N terminus stabilizes RhoA binding and inhibits RhoA activity. Consistent with this, increased expression of p120 isoform 1, when compared with other p120 isoforms, is predictive of renal tumor micrometastasis and systemic progression, following nephrectomy. Furthermore, ectopic expression of the RhoA-binding, N-terminal domain of p120 is sufficient to block the ability of p120 isoform 1 to inhibit RhoA and to promote invasiveness. The data indicate that the increased expression of p120 isoform 1 during tumor progression contributes to the invasive phenotype of cadherin-deficient carcinomas and that the N-terminal domain of p120 is a valid therapeutic target.
Figures

References
-
- Thiery, J. P. (2002) Nat. Rev Cancer 2 442-454 - PubMed
-
- Batlle, E., Sancho, E., Franci, C., Dominguez, D., Monfar, M., Baulida, J., and Garcia De Herreros, A. (2000) Nat. Cell Biol. 2 84-89 - PubMed
-
- Cano, A., Perez-Moreno, M. A., Rodrigo, I., Locascio, A., Blanco, M. J., del Barrio, M. G., Portillo, F., and Nieto, M. A. (2000) Nat. Cell Biol. 2 76-83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

