Linking functionally related genes by sensitive and quantitative characterization of genetic interaction profiles
- PMID: 18408161
- PMCID: PMC2311358
- DOI: 10.1073/pnas.0710533105
Linking functionally related genes by sensitive and quantitative characterization of genetic interaction profiles
Abstract
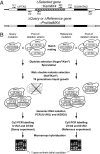
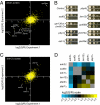
Describing at a genomic scale how mutations in different genes influence one another is essential to the understanding of how genotype correlates with phenotype and remains a major challenge in biology. Previous studies pointed out the need for accurate measurements of not only synthetic but also buffering interactions in the characterization of genetic networks and functional modules. We developed a sensitive and efficient method that allows such measurements at a genomic scale in yeast. In a pilot experiment (41 genome-wide screens), we quantified the fitness of 140,000 double deletion strains relative to the corresponding single mutants and identified many genetic interactions. In addition to synthetic growth defects (validated experimentally with factors newly identified as genetically interfering with mRNA degradation), most of the identified genetic interactions measured weak epistatic effects. These weak effects, rarely meaningful when considered individually, were crucial to defining specific signatures for many gene deletions and had a major contribution in defining clusters of functionally related genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Tong AH, et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. - PubMed
-
- Ooi SL, Shoemaker DD, Boeke JD. DNA helicase gene interaction network defined using synthetic lethality analyzed by microarray. Nat Genet. 2003;35:277–286. - PubMed
-
- Pan X, et al. A robust toolkit for functional profiling of the yeast genome. Mol Cell. 2004;16:487–496. - PubMed
-
- Tong AH, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. - PubMed
-
- Collins SR, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

