Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane
- PMID: 18408164
- PMCID: PMC2329711
- DOI: 10.1073/pnas.0708354105
Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane
Abstract
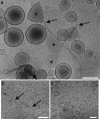
Fibrillar protein deposits (amyloid) in the pancreatic islets of Langerhans are thought to be involved in death of the insulin-producing islet beta cells in type 2 diabetes mellitus. It has been suggested that the mechanism of this beta cell death involves membrane disruption by human islet amyloid polypeptide (hIAPP), the major constituent of islet amyloid. However, the molecular mechanism of hIAPP-induced membrane disruption is not known. Here, we propose a hypothesis that growth of hIAPP fibrils at the membrane causes membrane damage. We studied the kinetics of hIAPP-induced membrane damage in relation to hIAPP fibril growth and found that the kinetic profile of hIAPP-induced membrane damage is characterized by a lag phase and a sigmoidal transition, which matches the kinetic profile of hIAPP fibril growth. The observation that seeding accelerates membrane damage supports the hypothesis. In addition, variables that are well known to affect hIAPP fibril formation, i.e., the presence of a fibril formation inhibitor, hIAPP concentration, and lipid composition, were found to have the same effect on hIAPP-induced membrane damage. Furthermore, electron microscopy analysis showed that hIAPP fibrils line the surface of distorted phospholipid vesicles, in agreement with the notion that hIAPP fibril growth at the membrane and membrane damage are physically connected. Together, these observations point toward a mechanism in which growth of hIAPP fibrils, rather than a particular hIAPP species, is responsible for the observed membrane damage. This hypothesis provides an additional mechanism next to the previously proposed role of oligomers as the main cytotoxic species of amyloidogenic proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Höppener JWM, Ahrén B, Lips CJM. Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 2000;343:411–419. - PubMed
-
- Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem. 1996;271:1988–1992. - PubMed
-
- Anguiano M, Nowak RJ, Lansbury PT., Jr Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. - PubMed
-
- Porat Y, Kolusheva S, Jelinek R, Gazit E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochemistry. 2003;42:10971–10977. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

