A review of the fetal brain cytokine imbalance hypothesis of schizophrenia
- PMID: 18408229
- PMCID: PMC2728807
- DOI: 10.1093/schbul/sbn022
A review of the fetal brain cytokine imbalance hypothesis of schizophrenia
Abstract
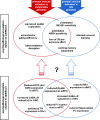
Maternal infection during pregnancy increases the risk of schizophrenia and other brain disorders of neurodevelopmental origin in the offspring. A multitude of infectious agents seem to be involved in this association. Therefore, it has been proposed that factors common to the immune response to a wide variety of bacterial and viral pathogens may be the critical link between prenatal infection and postnatal brain and behavioral pathology. More specifically, it has been suggested that the maternal induction of pro-inflammatory cytokines may mediate the neurodevelopmental effects of maternal infections. Here, we review recent findings from in vitro and in vivo investigations supporting this hypothesis and further emphasize the influence of enhanced anti-inflammatory cytokine signaling on early brain development. Disruption of the fetal brain balance between pro- and anti-inflammatory cytokine signaling may thus represent a key mechanism involved in the precipitation of schizophrenia-related pathology following prenatal maternal infection and innate immune imbalances.
Figures

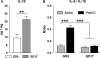
References
-
- Rees S, Harding R. Brain development during fetal life: influences of the intra-uterine environment. Neurosci Lett. 2004;361:111–114. - PubMed
-
- Rees S, Inder T. Fetal and neonatal origins of altered brain development. Early Hum Dev. 2005;81:753–761. - PubMed
-
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. - PubMed
-
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. - PubMed
-
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

