Mycoplasma infection suppresses p53, activates NF-kappaB and cooperates with oncogenic Ras in rodent fibroblast transformation
- PMID: 18408766
- PMCID: PMC4526267
- DOI: 10.1038/onc.2008.103
Mycoplasma infection suppresses p53, activates NF-kappaB and cooperates with oncogenic Ras in rodent fibroblast transformation
Abstract
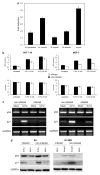
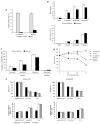
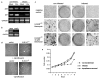
Prokaryotes of the genus Mycoplasma are the smallest cellular organisms that persist as obligate extracellular parasites. Although mycoplasma infection is known to be associated with chromosomal instability and can promote malignant transformation, the mechanisms underlying these phenomena remain unknown. Since persistence of many cellular parasites requires suppression of apoptosis in host cells, we tested the effect of mycoplasma infection on the activity of the p53 and nuclear factor (NF)-kappaB pathways, major mechanisms controlling programmed cell death. To monitor the activity of p53 and NF-kappaB in mycoplasma-infected cells, we used a panel of reporter cell lines expressing the bacterial beta-galactosidase gene under the control of p53- or NF-kappaB-responsive promoters. Cells incubated with media conditioned with different species of mycoplasma showed constitutive activation of NF-kappaB and reduced activation of p53, common characteristics of the majority of human tumor cells, with M. arginini having the strongest effect among the species tested. Moreover, mycoplasma infection reduced the expression level and inducibility of an endogenous p53-responsive gene, p21(waf1), and inhibited apoptosis induced by genotoxic stress. Infection with M. arginini made rat and mouse embryo fibroblasts susceptible to transformation with oncogenic H-Ras, whereas mycoplasma-free cells underwent irreversible p53-dependent growth arrest. Mycoplasma infection was as effective as shRNA-mediated knockdown of p53 expression in making rodent fibroblasts permissive to Ras-induced transformation. These observations indicate that mycoplasma infection plays the role of a p53-suppressing oncogene that cooperates with Ras in cell transformation and suggest that the carcinogenic and mutagenic effects of mycoplasma might be due to inhibition of p53 tumor suppressor function by this common human parasite.
Figures
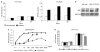
Similar articles
-
Activated oncogenes promote and cooperate with chromosomal instability for neoplastic transformation.Genes Dev. 2004 Jun 1;18(11):1317-30. doi: 10.1101/gad.1165204. Genes Dev. 2004. PMID: 15175263 Free PMC article.
-
Cooperation between p21 and Akt is required for p53-dependent cellular senescence.Aging Cell. 2017 Oct;16(5):1094-1103. doi: 10.1111/acel.12639. Epub 2017 Jul 9. Aging Cell. 2017. PMID: 28691365 Free PMC article.
-
Deregulation of p53/p21Cip1/Waf1 pathway contributes to polyploidy and apoptosis of E1A+cHa-ras transformed cells after gamma-irradiation.Oncogene. 1999 Oct 7;18(41):5611-9. doi: 10.1038/sj.onc.1202945. Oncogene. 1999. PMID: 10523840
-
Effects of mycoplasma infection on the host organism response via p53/NF-κB signaling.J Cell Physiol. 2018 Jan;234(1):171-180. doi: 10.1002/jcp.26781. Epub 2018 Aug 26. J Cell Physiol. 2018. PMID: 30146800 Review.
-
Role of Mycoplasma Chaperone DnaK in Cellular Transformation.Int J Mol Sci. 2020 Feb 15;21(4):1311. doi: 10.3390/ijms21041311. Int J Mol Sci. 2020. PMID: 32075244 Free PMC article. Review.
Cited by
-
p53 and the Carcinogenicity of Chronic Inflammation.Cold Spring Harb Perspect Med. 2016 Nov 1;6(11):a026161. doi: 10.1101/cshperspect.a026161. Cold Spring Harb Perspect Med. 2016. PMID: 27549311 Free PMC article. Review.
-
Role of Mycoplasma in the Initiation and Progression of Oral Cancer.J Int Oral Health. 2015 Jul;7(7):i-ii. J Int Oral Health. 2015. PMID: 26229390 Free PMC article. No abstract available.
-
Phenotypic characterization of Mycoplasma synoviae induced changes in the metabolic and sensitivity profile of in vitro infected chicken chondrocytes.Biomed Res Int. 2014;2014:613730. doi: 10.1155/2014/613730. Epub 2014 Aug 26. Biomed Res Int. 2014. PMID: 25243158 Free PMC article.
-
Potential malignant transformation in the gastric mucosa of immunodeficient mice with persistent Mycoplasma penetrans infection.PLoS One. 2017 Jul 10;12(7):e0180514. doi: 10.1371/journal.pone.0180514. eCollection 2017. PLoS One. 2017. Retraction in: PLoS One. 2020 Oct 22;15(10):e0241463. doi: 10.1371/journal.pone.0241463. PMID: 28692662 Free PMC article. Retracted.
-
The Role of the Microbiome in Oral Squamous Cell Carcinoma with Insight into the Microbiome-Treatment Axis.Int J Mol Sci. 2020 Oct 29;21(21):8061. doi: 10.3390/ijms21218061. Int J Mol Sci. 2020. PMID: 33137960 Free PMC article. Review.
References
-
- Baek KH, Shin HJ, Yoo JK, Cho JH, Choi YH, Sung YC, et al. p53 deficiency and defective mitotic checkpoint in proliferating T lymphocytes increase chromosomal instability through aberrant exit from mitotic arrest. J Leukoc Biol. 2003;73:850–861. - PubMed
-
- Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol Histopathol. 2006;21:69–80. - PubMed
-
- Burgert HG, Ruzsics Z, Obermeier S, Hilgendorf A, Windheim M, Elsing A. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318. - PubMed
-
- Chu HW, Jeyaseelan S, Rino JG, Voelker DR, Wexler RB, Campbell K, et al. TLR2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J Immunol. 2005;9:5713–5719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

