Using the zebrafish lateral line to screen for ototoxicity
- PMID: 18408970
- PMCID: PMC2504598
- DOI: 10.1007/s10162-008-0118-y
Using the zebrafish lateral line to screen for ototoxicity
Abstract
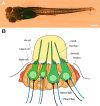
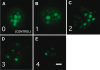
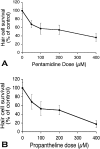
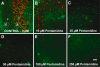
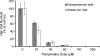
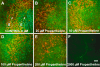
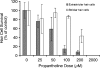
The zebrafish is a valuable model for studying hair cell development, structure, genetics, and behavior. Zebrafish and other aquatic vertebrates have hair cells on their body surface organized into a sensory system called the lateral line. These hair cells are highly accessible and easily visualized using fluorescent dyes. Morphological and functional similarities to mammalian hair cells of the inner ear make the zebrafish a powerful preparation for studying hair cell toxicity. The ototoxic potential of drugs has historically been uncovered by anecdotal reports that have led to more formal investigation. Currently, no standard screen for ototoxicity exists in drug development. Thus, for the vast majority of Food and Drug Association (FDA)-approved drugs, the ototoxic potential remains unknown. In this study, we used 5-day-old zebrafish larvae to screen a library of 1,040 FDA-approved drugs and bioactives (NINDS Custom Collection II) for ototoxic effects in hair cells of the lateral line. Hair cell nuclei were selectively labeled using a fluorescent vital dye. For the initial screen, fish were exposed to drugs from the library at a 100-muM concentration for 1 h in 96-well tissue culture plates. Hair cell viability was assessed in vivo using fluorescence microscopy. One thousand forty drugs were rapidly screened for ototoxic effects. Seven known ototoxic drugs included in the library, including neomycin and cisplatin, were positively identified using these methods, as proof of concept. Fourteen compounds without previously known ototoxicity were discovered to be selectively toxic to hair cells. Dose-response curves for all 21 ototoxic compounds were determined by quantifying hair cell survival as a function of drug concentration. Dose-response relationships in the mammalian inner ear for two of the compounds without known ototoxicity, pentamidine isethionate and propantheline bromide, were then examined using in vitro preparations of the adult mouse utricle. Significant dose-dependent hair cell loss in the mouse utricle was demonstrated for both compounds. This study represents an important step in validating the use of the zebrafish lateral line as a screening tool for the identification of potentially ototoxic drugs.
Figures
References
-
- Bellamy R. Pneumocystis pneumonia in people with HIV. Clin. Evid. 13:854–868, 2006. - PubMed
-
- Calza L, Marinacci G, Manfredi R, Colangeli V, Fortunat L, Chiodo F. Pentamidine isethionate as treatment and secondary prophylaxis for disseminated cutaneous leishmaniasis during HIV infection: case report. J. Chemother. 13:653–657, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

