Ring size of somatostatin analogues (ODT-8) modulates receptor selectivity and binding affinity
- PMID: 18410084
- PMCID: PMC2782568
- DOI: 10.1021/jm701444y
Ring size of somatostatin analogues (ODT-8) modulates receptor selectivity and binding affinity
Abstract
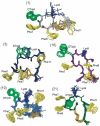
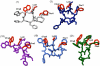
The synthesis, biological testing, and NMR studies of several analogues of H-c[Cys (3)-Phe (6)-Phe (7)-DTrp (8)-Lys (9)-Thr (10)-Phe (11)-Cys (14)]-OH (ODT-8, a pan-somatostatin analogue, 1) have been performed to assess the effect of changing the stereochemistry and the number of atoms in the disulfide bridge on binding affinity. Cysteine at positions 3 and/or 14 (somatostatin numbering) were/was substituted with d-cysteine, norcysteine, D-norcysteine, homocysteine, and/or D-homocysteine. The 3D structure analysis of selected partially selective, bioactive analogues (3, 18, 19, and 21) was carried out in dimethylsulfoxide. Interestingly and not unexpectedly, the 3D structures of these analogues comprised the pharmacophore for which the analogues had the highest binding affinities (i.e., sst 4 in all cases).
Figures

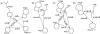
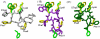
Similar articles
-
Novel sst(4)-selective somatostatin (SRIF) agonists. 1. Lead identification using a betide scan.J Med Chem. 2003 Dec 18;46(26):5579-86. doi: 10.1021/jm030243c. J Med Chem. 2003. PMID: 14667212
-
Novel sst(4)-selective somatostatin (SRIF) agonists. 3. Analogues amenable to radiolabeling.J Med Chem. 2003 Dec 18;46(26):5597-605. doi: 10.1021/jm030245x. J Med Chem. 2003. PMID: 14667214
-
Novel sst(4)-selective somatostatin (SRIF) agonists. 2. Analogues with beta-methyl-3-(2-naphthyl)alanine substitutions at position 8.J Med Chem. 2003 Dec 18;46(26):5587-96. doi: 10.1021/jm0302445. J Med Chem. 2003. PMID: 14667213
-
Ring size in octreotide amide modulates differently agonist versus antagonist binding affinity and selectivity.J Med Chem. 2008 May 8;51(9):2676-81. doi: 10.1021/jm701445q. Epub 2008 Apr 12. J Med Chem. 2008. PMID: 18410083 Free PMC article.
-
Novel sst(4)-selective somatostatin (SRIF) agonists. 4. Three-dimensional consensus structure by NMR.J Med Chem. 2003 Dec 18;46(26):5606-18. doi: 10.1021/jm030246p. J Med Chem. 2003. PMID: 14667215
Cited by
-
The role of brain somatostatin receptor 2 in the regulation of feeding and drinking behavior.Horm Behav. 2015 Jul;73:15-22. doi: 10.1016/j.yhbeh.2015.05.009. Epub 2015 May 27. Horm Behav. 2015. PMID: 26026616 Free PMC article. Review.
-
Central actions of somatostatin-28 and oligosomatostatin agonists to prevent components of the endocrine, autonomic and visceral responses to stress through interaction with different somatostatin receptor subtypes.Curr Pharm Des. 2013;19(1):98-105. doi: 10.2174/13816128130114. Curr Pharm Des. 2013. PMID: 22950508 Free PMC article. Review.
-
Three-dimensional consensus structure of sst2-selective somatostatin (SRIF) antagonists by NMR.Biopolymers. 2008 Dec;89(12):1077-87. doi: 10.1002/bip.21060. Biopolymers. 2008. PMID: 18655144 Free PMC article.
-
Activation of brain somatostatin 2 receptors stimulates feeding in mice: analysis of food intake microstructure.Physiol Behav. 2010 Dec 2;101(5):614-22. doi: 10.1016/j.physbeh.2010.09.009. Epub 2010 Sep 17. Physiol Behav. 2010. PMID: 20851136 Free PMC article.
-
Orexigenic response to tail pinch: role of brain NPY(1) and corticotropin releasing factor receptors.Am J Physiol Regul Integr Comp Physiol. 2014 Feb 1;306(3):R164-74. doi: 10.1152/ajpregu.00335.2013. Epub 2013 Dec 11. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24338440 Free PMC article.
References
-
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. - PubMed
-
- Guillemin R, Gerich JE. Somatostatin: physiological and clinical significance. Ann. Rev. Med. 1976;27:379–388. - PubMed
-
- Reubi JC, Schaer JC, Waser B, Wenger S, Heppeler A, Schmitt JS, Mäcke HR. Affinity profiles for human somatostatin receptor sst1-sst5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000;27:273–282. - PubMed
-
- Janecka A, Zubrzycka M, Janecki T. Review: Somatostatin analogs. J. Pept. Res. 2001;58:91–107. - PubMed
-
- Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga JL, Halmos G. Hypothalamic hormones and cancer. Front. Neuroendocrinol. 2001;22:248–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

