Synthesis and structure verification of the vaccine adjuvant QS-7-Api. Synthetic access to homogeneous Quillaja saponaria immunostimulants
- PMID: 18410100
- PMCID: PMC2610460
- DOI: 10.1021/ja801008m
Synthesis and structure verification of the vaccine adjuvant QS-7-Api. Synthetic access to homogeneous Quillaja saponaria immunostimulants
Abstract
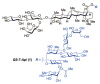
QS-7-Api is an exceedingly potent immuno-adjuvant isolated from the bark of Quillaja saponaria. It is significantly less toxic than QS-21, a related saponin that is currently the favored adjuvant in anticancer and antiviral vaccine clinical trials. Tedious isolation/purification protocols and uncertainty in its structural constitution have hindered the clinical development of QS-7. A chemical synthesis of QS-7-Api is described, providing structural verification of the adjuvant. A novel semisynthetic sequence to QS-7-Api has also been established, greatly facilitating access to QS-7 for preclinical and clinical evaluation.
Figures



References
-
- Kensil CR, Patel U, Lennick M, Marciani D. J Immunol. 1991;146:431–437. - PubMed
-
- Soltysik S, Wu JY, Recchia J, Wheeler DA, Newman MJ, Coughlin RT, Kensil CR. Vaccine. 1995;13:1403–1410. - PubMed
-
- Kensil CR. Crit Rev Ther Drug Carrier Syst. 1996;13:1–55. - PubMed
-
- Kim SK, Ragupathi G, Musselli C, Choi SJ, Park YS, Livingston PO. Vaccine. 2000;18:597–603. - PubMed
-
- Livingston PO, Ragupathi G. Hum Vaccines. 2006;2:137–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

