The binding and release of oxygen and hydrogen peroxide are directed by a hydrophobic tunnel in cholesterol oxidase
- PMID: 18410129
- PMCID: PMC2561232
- DOI: 10.1021/bi800228w
The binding and release of oxygen and hydrogen peroxide are directed by a hydrophobic tunnel in cholesterol oxidase
Abstract
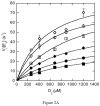
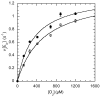
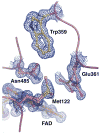
The usage by enzymes of specific binding pathways for gaseous substrates or products is debated. The crystal structure of the redox enzyme cholesterol oxidase, determined at sub-angstrom resolution, revealed a hydrophobic tunnel that may serve as a binding pathway for oxygen and hydrogen peroxide. This tunnel is formed by a cascade of conformational rearrangements and connects the active site with the exterior surface of the protein. To elucidate the relationship between this tunnel and gas binding and release, three mutant enzymes were constructed to block the tunnel or its putative gate. Mutation of the proposed gating residue Asn485 to Asp or tunnel residue Phe359 or Gly347 to Trp or Asn reduces the catalytic efficiency of oxidation. The K mO 2 increases from 300 +/- 35 microM for the wild-type enzyme to 617 +/- 15 microM for the F359W mutant. The k cat for the F359W mutant-catalyzed reaction decreases 13-fold relative to that of the wild-type-catalyzed reaction. The N485D and G347N mutants could not be saturated with oxygen. Transfer of hydride from the sterol to the flavin prosthetic group is no longer rate-limiting for these tunnel mutants. The steady-state kinetics of both wild-type and tunnel mutant enzymes are consistent with formation of a ternary complex of steroid and oxygen during catalysis. Furthermore, kinetic cooperativity with respect to molecular oxygen is observed with the tunnel mutants, but not with the wild-type enzyme. A rate-limiting conformational change for binding and release of oxygen and hydrogen peroxide, respectively, is consistent with the cooperative kinetics. In the atomic-resolution structure of F359W, the indole ring of the tryptophan completely fills the tunnel and is observed in only a single conformation. The size of the indole is proposed to limit conformational rearrangement of residue 359 that leads to tunnel opening in the wild-type enzyme. Overall, these results substantiate the functional importance of the tunnel for substrate binding and product release.
Figures






References
-
- Tilton RF, Jr, Kuntz ID, Jr, Petsko GA. Cavities in proteins: structure of a metmyoglobin-xenon complex solved to 1.9 A. Biochemistry. 1984;23:2849–2857. - PubMed
-
- Whittington DA, Rosenzweig AC, Frederick CA, Lippard SJ. Xenon and halogenated alkanes track putative substrate binding cavities in the soluble methane monooxygenase hydroxylase. Biochemistry. 2001;40:3476–3482. - PubMed
-
- Duff AP, Trambaiolo DM, Cohen AE, Ellis PJ, Juda GA, Shepard EM, Langley DB, Dooley DM, Freeman HC, Guss JM. Using xenon as a probe for dioxygen-binding sites in copper amine oxidases. J Mol Biol. 2004;344:599–607. - PubMed
-
- de Sanctis D, Dewilde S, Pesce A, Moens L, Ascenzi P, Hankeln T, Burmester T, Bolognesi M. Mapping protein matrix cavities in human cytoglobin through Xe atom binding. Biochem Biophys Res Commun. 2004;316:1217–1221. - PubMed
-
- Raushel FM, Thoden JB, Holden HM. Enzymes with molecular tunnels. Acc Chem Res. 2003;36:539–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

