Ultraviolet C inactivation of dermatophytes: implications for treatment of onychomycosis
- PMID: 18410410
- PMCID: PMC2808700
- DOI: 10.1111/j.1365-2133.2008.08549.x
Ultraviolet C inactivation of dermatophytes: implications for treatment of onychomycosis
Abstract
Background: Onychomycosis responds to systemic antifungals and sometimes to topical lacquers, but alternative treatments are desirable. Topical application of germicidal ultraviolet (UV) C radiation may be an acceptable and effective therapy for infected nails.
Objectives: To test the ability of UVC to inactivate dermatophyte suspensions in vitro and to sterilize a novel ex vivo model of nail infection.
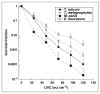
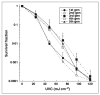
Methods: Trichophyton rubrum, T. mentagrophytes, Epidermophyton floccosum and Microsporum canis suspensions were irradiated with UVC (254 nm) at a radiant exposure of 120 mJ cm(-2) and surviving colony-forming units quantified. T. rubrum infecting porcine hoof slices and human toenail clippings was irradiated with UVC at radiant exposures of 36-864 J cm(-2).
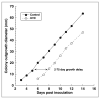
Results: In vitro studies showed that 3-5 logs of cell inactivation in dermatophyte suspensions were produced with 120 mJ cm(-2) UVC irradiation. Depending on factors such as the thickness and infectious burden of the ex vivo cultures, the radiant exposure of UVC needed for complete sterilization was usually in the order of tens to hundreds of J cm(-2). Resistance of T. rubrum to UVC irradiation did not increase after five cycles of subtotal inactivation in vitro.
Conclusions: UVC irradiation may be a less invasive treatment option for onychomycosis, when the appropriate consideration is given to safety.
Conflict of interest statement
Figures



Similar articles
-
An investigation into the inhibitory effect of ultraviolet radiation on Trichophyton rubrum.Lasers Med Sci. 2014 Jan;29(1):157-63. doi: 10.1007/s10103-013-1287-4. Epub 2013 Mar 23. Lasers Med Sci. 2014. PMID: 23525830
-
Ex vivo potential of a quinoline-derivative nail lacquer as a new alternative for dermatophytic onychomycosis treatment.J Med Microbiol. 2021 Mar;70(3). doi: 10.1099/jmm.0.001314. Epub 2021 Jan 27. J Med Microbiol. 2021. PMID: 33502306
-
Five-hour diagnosis of dermatophyte nail infections with specific detection of Trichophyton rubrum.J Clin Microbiol. 2007 Apr;45(4):1200-4. doi: 10.1128/JCM.02072-06. Epub 2007 Jan 31. J Clin Microbiol. 2007. PMID: 17267633 Free PMC article.
-
[Antimycotic therapy of Tinea unguium and other onychomycoses].Med Monatsschr Pharm. 2009 Aug;32(8):289-98; quiz 299-300. Med Monatsschr Pharm. 2009. PMID: 19777736 Review. German.
-
Efficacy of Lasers for the Management of Dermatophyte Toenail Onychomycosis.J Am Podiatr Med Assoc. 2022 Mar 16;112(1):20-236. doi: 10.7547/20-236. J Am Podiatr Med Assoc. 2022. PMID: 34233353 Review.
Cited by
-
Effects of UVC Irradiation on Growth and Apoptosis of Scedosporium apiospermum and Lomentospora prolificans.Interdiscip Perspect Infect Dis. 2018 Dec 2;2018:3748594. doi: 10.1155/2018/3748594. eCollection 2018. Interdiscip Perspect Infect Dis. 2018. PMID: 30631350 Free PMC article.
-
The Dermatologist's Approach to Onychomycosis.J Fungi (Basel). 2015 Aug 19;1(2):173-184. doi: 10.3390/jof1020173. J Fungi (Basel). 2015. PMID: 29376907 Free PMC article. Review.
-
Extreme sensitivity to ultraviolet light in the fungal pathogen causing white-nose syndrome of bats.Nat Commun. 2018 Jan 2;9(1):35. doi: 10.1038/s41467-017-02441-z. Nat Commun. 2018. PMID: 29295979 Free PMC article.
-
Fungal photoinactivation doses for UV radiation and visible light-a data collection.AIMS Microbiol. 2024 Aug 22;10(3):694-722. doi: 10.3934/microbiol.2024032. eCollection 2024. AIMS Microbiol. 2024. PMID: 39219750 Free PMC article. Review.
-
UVC light prophylaxis for cutaneous wound infections in mice.Antimicrob Agents Chemother. 2012 Jul;56(7):3841-8. doi: 10.1128/AAC.00161-12. Epub 2012 May 7. Antimicrob Agents Chemother. 2012. PMID: 22564833 Free PMC article.
References
-
- Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172–3. - PubMed
-
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641–8. - PubMed
-
- Robbins JM. Treatment of onychomycosis in the diabetic patient population. J Diabetes Complications. 2003;17:98–104. - PubMed
-
- Elewski BE, Hay RJ. Update on the management of onychomycosis: highlights of the Third Annual International Summit on Cutaneous Antifungal Therapy. Clin Infect Dis. 1996;23:305–13. - PubMed
-
- Clayton YM. Clinical and mycological diagnostic aspects of onychomycoses and dermatomycoses. Clin Exp Dermatol. 1992;17(Suppl 1):37–40. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

