TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease
- PMID: 18411337
- PMCID: PMC2373838
- DOI: 10.1084/jem.20071364
TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease
Abstract
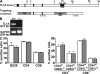
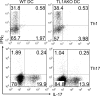
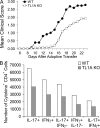
T helper type 17 (Th17) cells play an important pathogenic function in autoimmune diseases; their regulation, however, is not well understood. We show that the expression of a tumor necrosis factor receptor family member, death receptor 3 (DR3; also known as TNFRSF25), is selectively elevated in Th17 cells, and that TL1A, its cognate ligand, can promote the proliferation of effector Th17 cells. To further investigate the role of the TL1A-DR3 pathway in Th17 regulation, we generated a TL1A-deficient mouse and found that TL1A(-/-) dendritic cells exhibited a reduced capacity in supporting Th17 differentiation and proliferation. Consistent with these data, TL1A(-/-) animals displayed decreased clinical severity in experimental autoimmune encephalomyelitis (EAE). Finally, we demonstrated that during EAE disease progression, TL1A was required for the optimal differentiation as well as effector function of Th17 cells. These observations thus establish an important role of the TL1A-DR3 pathway in promoting Th17 cell function and Th17-mediated autoimmune disease.
Figures
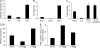





References
-
- Glimcher, L.H., and K.M. Murphy. 2000. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14:1693–1711. - PubMed
-
- Dong, C. 2006. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 6:329–333. - PubMed
-
- Weaver, C.T., L.E. Harrington, P.R. Mangan, M. Gavrieli, and K.M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24:677–688. - PubMed
-
- Bettelli, E., M. Oukka, and V.K. Kuchroo. 2007. TH-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

