Design and microwave-assisted synthesis of novel macrocyclic peptides active at melanocortin receptors: discovery of potent and selective hMC5R receptor antagonists
- PMID: 18412316
- PMCID: PMC2756086
- DOI: 10.1021/jm701181n
Design and microwave-assisted synthesis of novel macrocyclic peptides active at melanocortin receptors: discovery of potent and selective hMC5R receptor antagonists
Abstract
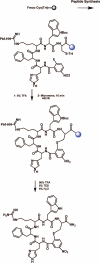
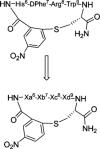
Differentiation of the physiological role of the melanocortin receptor 5 MC5R from that of other melanocortin receptors will require development of high affinity and selective antagonists. To date, a few synthetic antagonist ligands active at hMC5 receptor are available, but most do not have appreciable selectivity. With the aim to gain more potent and selective antagonists for the MC5R ligands, we have designed, synthesized, and pharmacologically characterized a series of alkylthioaryl-bridged macrocyclic peptide analogues derived from MT-II and SHU9119. These 20-membered macrocycles were synthesized by a tandem combination using solid phase peptide synthesis and microwave-assisted reactions. Biological assays for binding affinities and adenylate cyclase activities for the hMC1R, hMC3R, hMC4R, and hMC5R showed that three analogues, compounds, 9, 4, and 7, are selective antagonists at the hMC5 receptor. In particular, compound 9(PG-20N) is a selective and competitive hMC5R antagonist, with IC 50 of 130 +/- 11 nM, and a pA 2 value of 8.3, and represents an important tool for further biological investigations of the hMC5R. Compounds 4 and 7 (PG14N, PG17N) show potent and selective allosteric inhibition at hMC5R with IC 50 values of 38 +/- 3 nM and 58 +/- 6 nM, respectively. Compound 9 will be used to further investigate and more clearly understand the physiological roles played by the MC5 receptor in humans and other animals.
Figures
Similar articles
-
Development of cyclic gamma-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities.J Med Chem. 2006 Mar 23;49(6):1946-52. doi: 10.1021/jm0510326. J Med Chem. 2006. PMID: 16539382 Free PMC article.
-
Structure-activity studies of the melanocortin peptides: discovery of potent and selective affinity antagonists for the hMC3 and hMC4 receptors.J Med Chem. 2002 Nov 21;45(24):5287-94. doi: 10.1021/jm0202526. J Med Chem. 2002. PMID: 12431055
-
Design, synthesis, and biological evaluation of new cyclic melanotropin peptide analogues selective for the human melanocortin-4 receptor.J Med Chem. 2006 Nov 16;49(23):6888-96. doi: 10.1021/jm060768f. J Med Chem. 2006. PMID: 17154518 Free PMC article.
-
Design of novel melanotropin agonists and antagonists with high potency and selectivity for human melanocortin receptors.Peptides. 2005 Aug;26(8):1481-5. doi: 10.1016/j.peptides.2005.03.020. Peptides. 2005. PMID: 15876475 Review.
-
Design of cyclic peptides with biological activities from biologically active peptides: the case of peptide modulators of melanocortin receptors.Biopolymers. 2016 Nov;106(6):884-888. doi: 10.1002/bip.22929. Biopolymers. 2016. PMID: 27486849 Free PMC article. Review.
Cited by
-
Region-specific differences in brain melanocortin receptors in rats of the lean phenotype.Neuroreport. 2012 Jul 11;23(10):596-600. doi: 10.1097/WNR.0b013e328354f5c1. Neuroreport. 2012. PMID: 22643233 Free PMC article.
-
Activation of Melanocortin Receptors MC 1 and MC 5 Attenuates Retinal Damage in Experimental Diabetic Retinopathy.Mediators Inflamm. 2016;2016:7368389. doi: 10.1155/2016/7368389. Epub 2016 Jan 12. Mediators Inflamm. 2016. PMID: 26949291 Free PMC article.
-
Development of Macrocyclic Peptidomimetics Containing Constrained α,α-Dialkylated Amino Acids with Potent and Selective Activity at Human Melanocortin Receptors.J Med Chem. 2018 May 10;61(9):4263-4269. doi: 10.1021/acs.jmedchem.8b00488. Epub 2018 Apr 25. J Med Chem. 2018. PMID: 29660981 Free PMC article.
-
Development of a Backbone Cyclic Peptide Library as Potential Antiparasitic Therapeutics Using Microwave Irradiation.J Vis Exp. 2016 Jan 26;(107):e53589. doi: 10.3791/53589. J Vis Exp. 2016. PMID: 26863382 Free PMC article.
-
Old drugs with new skills: fenoprofen as an allosteric enhancer at melanocortin receptor 3.Cell Mol Life Sci. 2017 Apr;74(7):1335-1345. doi: 10.1007/s00018-016-2419-3. Epub 2016 Nov 16. Cell Mol Life Sci. 2017. PMID: 27853832 Free PMC article.
References
-
- Mains RE, Eipper BA. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J. Biol. Chem. 1976;251:4115–4120. - PubMed
-
- Eipper BA, Mains RE. Structure and biosynthesis of proadrenocorticotropin/endorphin and related peptides. Endocr. Rev. 1980;1:1–27. - PubMed
-
- Hadley ME. Endocrinology. 4th edition Prentice-Hall; Upper Saddle River, N.J.: 1996.
-
- Simpson ER, Waterman MR. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu. Rev. Physiol. 1988;50:427–440. - PubMed
-
- Geschwind II, Huseby RA, Nishioka R. The effect of melanocyte-stimulating hormone on coat color in the mouse. Recent Prog. Horm. Res. 1972;28:91–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

