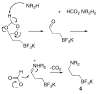
Functionalization of organotrifluoroborates: reductive amination
- PMID: 18412389
- PMCID: PMC2819055
- DOI: 10.1021/jo800383e
Functionalization of organotrifluoroborates: reductive amination
Abstract
Herein we report the conversion of aldehyde-containing potassium and tetrabutylammonium organotrifluoroborates to the corresponding amines through reductive amination protocols. Potassium formate facilitated by catalytic palladium acetate, sodium triacetoxyborohydride, and pyridine borane have all served as effective hydride donors, reducing the initially formed imines or iminium ions to provide the corresponding amines.
References
-
- Molander GA, Ellis N. Acc Chem Res. 2007;40:275–286. - PubMed
-
- Molander GA, Ribagorda M. J Am Chem Soc. 2003;125:11148–11149. - PubMed
- Molander GA, Ellis NM. J Org Chem. 2006;71:7491–7493. - PMC - PubMed
- Molander GA, Figueroa R. Org Lett. 2006;8:75–78. - PMC - PubMed
- Molander GA, Figueroa R. J Org Chem. 2006;71:6135–6149. - PubMed
- Molander GA, Cooper DJ. J Org Chem. 2007;72:3558–3560. - PMC - PubMed
-
- Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA. Org Chem. 1996;61:3849–3862. - PubMed
- Abdel-Magid AF, Mehrman SJ. Org Process Rev Dev. 2006;10:971–1031.
- Quan ML, Han Q, Fevig JM, Lam PYS, Bai S, Knabb RM, Luettgen JM, Wong PC, Wexler RR. Bioorg Med Chem Lett. 2006;16:1795–1798. - PubMed
- Baxter EW, Reitz AB. Organic Reactions. Vol. 59 John Wiley and Sons, Inc.; New York: 2002.
-
- Schwartz C, Raible J, Mott K, Dussault PH. Org Lett. 2006;8:3199–3201. - PubMed
-
- Leuckart R. J Prakt Chem. 1890;41:33.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases