Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy
- PMID: 18412402
- PMCID: PMC3952070
- DOI: 10.1021/nl080496z
Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy
Abstract
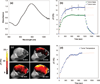
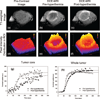
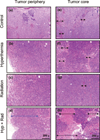
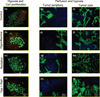
We report noninvasive modulation of in vivo tumor radiation response using gold nanoshells. Mild-temperature hyperthermia generated by near-infrared illumination of gold nanoshell-laden tumors, noninvasively quantified by magnetic resonance temperature imaging, causes an early increase in tumor perfusion that reduces the hypoxic fraction of tumors. A subsequent radiation dose induces vascular disruption with extensive tumor necrosis. Gold nanoshells sequestered in the perivascular space mediate these two tumor vasculature-focused effects to improve radiation response of tumors. This novel integrated antihypoxic and localized vascular disrupting therapy can potentially be combined with other conventional antitumor therapies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

