Neuroplasticity in brain reward circuitry following a history of ethanol dependence
- PMID: 18412612
- PMCID: PMC2486413
- DOI: 10.1111/j.1460-9568.2008.06159.x
Neuroplasticity in brain reward circuitry following a history of ethanol dependence
Abstract
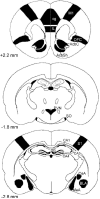
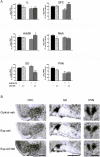
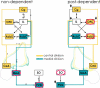
Mitogen-activated and extracellular regulated kinase (MEK) and extracellular signal-regulated protein kinase (ERK) pathways may underlie ethanol-induced neuroplasticity. Here, we used the MEK inhibitor 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (UO126) to probe the role of MEK/ERK signaling for the cellular response to an acute ethanol challenge in rats with or without a history of ethanol dependence. Ethanol (1.5 g/kg, i.p.) induced expression of the marker genes c-fos and egr-1 in brain regions associated with both rewarding and stressful ethanol actions. Under non-dependent conditions, ethanol-induced c-fos expression was generally not affected by MEK inhibition, with the exception of the medial amygdala (MeA). In contrast, following a history of dependence, a markedly suppressed c-fos response to acute ethanol was found in the medial pre-frontal/orbitofrontal cortex (OFC), nucleus accumbens shell (AcbSh) and paraventricular nucleus (PVN). The suppressed ethanol response in the OFC and AcbSh, key regions involved in ethanol preference and seeking, was restored by pre-treatment with UO126, demonstrating a recruitment of an ERK/MEK-mediated inhibitory regulation in the post-dependent state. Conversely, in brain areas involved in stress responses (MeA and PVN), an MEK/ERK-mediated cellular activation by acute ethanol was lost following a history of dependence. These data reveal region-specific neuroadaptations encompassing the MEK/ERK pathway in ethanol dependence. Recruitment of MEK/ERK-mediated suppression of the ethanol response in the OFC and AcbSh may reflect devaluation of ethanol as a reinforcer, whereas loss of an MEK/ERK-mediated response in the MeA and PVN may reflect tolerance to its aversive actions. These two neuroadaptations could act in concert to facilitate progression into ethanol dependence.
Figures


Similar articles
-
Mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase (ERK) pathway.Mol Cancer Ther. 2002 Mar;1(5):303-9. Mol Cancer Ther. 2002. PMID: 12489846
-
Identification of a novel inhibitor of mitogen-activated protein kinase kinase.J Biol Chem. 1998 Jul 17;273(29):18623-32. doi: 10.1074/jbc.273.29.18623. J Biol Chem. 1998. PMID: 9660836
-
Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen.J Immunol. 2001 Mar 15;166(6):3855-64. doi: 10.4049/jimmunol.166.6.3855. J Immunol. 2001. PMID: 11238629
-
Pharmacologic inhibition of RAF-->MEK-->ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1.Cancer Res. 2005 Jun 1;65(11):4870-80. doi: 10.1158/0008-5472.CAN-04-2848. Cancer Res. 2005. PMID: 15930308
-
Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression.Alcohol Clin Exp Res. 2009 Jun;33(6):945-69. doi: 10.1111/j.1530-0277.2009.00916.x. Epub 2009 Mar 19. Alcohol Clin Exp Res. 2009. PMID: 19302091 Review.
Cited by
-
Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking.Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18156-61. doi: 10.1073/pnas.1116523109. Epub 2012 Oct 15. Proc Natl Acad Sci U S A. 2012. PMID: 23071333 Free PMC article.
-
Effects of ceftriaxone on ethanol drinking and GLT-1 expression in ethanol dependence and relapse drinking.Alcohol. 2021 May;92:1-9. doi: 10.1016/j.alcohol.2021.01.004. Epub 2021 Jan 16. Alcohol. 2021. PMID: 33465464 Free PMC article.
-
Increasing Brain-Derived Neurotrophic Factor (BDNF) in medial prefrontal cortex selectively reduces excessive drinking in ethanol dependent mice.Neuropharmacology. 2018 Sep 15;140:35-42. doi: 10.1016/j.neuropharm.2018.07.031. Epub 2018 Jul 26. Neuropharmacology. 2018. PMID: 30056122 Free PMC article.
-
The synaptoneurosome transcriptome: a model for profiling the emolecular effects of alcohol.Pharmacogenomics J. 2015 Apr;15(2):177-88. doi: 10.1038/tpj.2014.43. Epub 2014 Aug 19. Pharmacogenomics J. 2015. PMID: 25135349 Free PMC article.
-
Neuropeptide Y in Alcohol Addiction and Affective Disorders.Front Endocrinol (Lausanne). 2017 Jul 31;8:178. doi: 10.3389/fendo.2017.00178. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28824541 Free PMC article. Review.
References
-
- Alheid GF. Extended amygdala and basal forebrain. Amygdala in Brain Function: Bacic and Clinical Approaches. 2003;985:185–205. - PubMed
-
- Arlinde C, Sommer W, Bjork K, Reimers M, Hyytia P, Kiianmaa K, Heilig M. A cluster of differentially expressed signal transduction genes identified by microarray analysis in a rat genetic model of alcoholism. Pharmacogenomics J. 2004;4:208–218. - PubMed
-
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302:516–524. - PubMed
-
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: Effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci.Biobehav.Rev. 2005;29:1243–1253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

