Dendritic cells are preferentially targeted among hematolymphocytes by Modified Vaccinia Virus Ankara and play a key role in the induction of virus-specific T cell responses in vivo
- PMID: 18412969
- PMCID: PMC2359732
- DOI: 10.1186/1471-2172-9-15
Dendritic cells are preferentially targeted among hematolymphocytes by Modified Vaccinia Virus Ankara and play a key role in the induction of virus-specific T cell responses in vivo
Abstract
Background: Modified Vaccinia Ankara (MVA) is a highly attenuated strain of vaccinia virus (VV) that has lost approximately 15% of the VV genome, along with the ability to replicate in most mammalian cells. It has demonstrated impressive safety and immunogenicity profile in both preclinical and clinical studies, and is being actively explored as a promising vaccine vector for a number of infectious diseases and malignancies. However, little is known about how MVA interacts with the host immune system constituents, especially dendritic cells (DCs), to induce strong immune responses despite its inability to replicate in vivo. Using in vitro and in vivo murine models, we systematically investigated the susceptibility of murine DCs to MVA infection, and the immunological consequences of the infection.
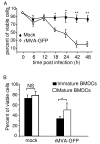
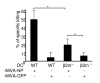
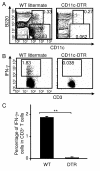
Results: Our data demonstrate that MVA preferentially infects professional antigen presenting cells, especially DCs, among all the subsets of hematolymphoid cells. In contrast to the reported blockage of DC maturation and function upon VV infection, DCs infected by MVA undergo phenotypic maturation and produce innate cytokine IFN-alpha within 18 h of infection. Substantial apoptosis of MVA-infected DCs occurs after 12 h following infection and the apoptotic DCs are readily phagocytosed by uninfected DCs. Using MHC class I - deficient mice, we showed that both direct and cross-presentation of viral Ags are likely to be involved in generating viral-specific CD8+ T cell responses. Finally, DC depletion abrogated the T cell activation in vivo.
Conclusion: We present the first in vivo evidence that among hematolymphoid cells, DCs are the most susceptible targets for MVA infection, and DC-mediated Ag presentation is required for the induction of MVA-specific immune responses. These results provide important information concerning the mechanisms by which strong immune responses are elicited to MVA-encoded antigens and may inform efforts to further improve the immunogenicity of this already promising vaccine vector.
Figures



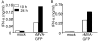

References
-
- Willis NJ. Edward Jenner and the eradication of smallpox. Scott Med J. 1997;42:118–121. - PubMed
-
- Neff JM, Levine RH, Lane JM, Ager EA, Moore H, Rosenstein BJ, Millar JD, Henderson DA. Complications of smallpox vaccination United States 1963. II. Results obtained by four statewide surveys. Pediatrics. 1967;39:916–923. - PubMed
-
- Neff JM, Lane JM, Pert JH, Moore R, Millar JD, Henderson DA. Complications of smallpox vaccination. I. National survey in the United States, 1963. New England Journal of Medicine. 1967;276:125–132. - PubMed
-
- Mayr A, Stickl H, Muller HK, Danner K, Singer H. [The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl)] Zentralbl Bakteriol [B] 1978;167:375–390. - PubMed
-
- Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72:1031–1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

