Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer
- PMID: 18413743
- PMCID: PMC2823123
- DOI: 10.1158/0008-5472.CAN-07-6349
Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer
Erratum in
- Cancer Res. 2008 Jul 15;68(14):6030
Abstract
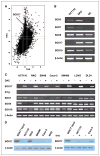
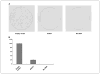
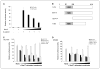
SRY-box containing gene 17 (Sox17) is a member of the high mobility group (HMG) transcription factor superfamily, which plays critical roles in the regulation of development and stem/precursor cell function, at least partly through repression of Wnt pathway activity. Modulators controlling aberrant Wnt signaling activation are frequently disrupted in human cancers through complementary effects of epigenetic and genetic changes. Our recent global analysis of CpG island hypermethylation and gene expression in colorectal cancer (CRC) cell lines revealed that SOX17 gene silencing is associated with DNA hypermethylation of a CpG island in the promoter region. Here, we report that CpG island methylation-dependent silencing of SOX17 occurs in 100% of CRC cell lines, 86% of colorectal adenomas, 100% of stage I and II CRC, 89% of stage III CRC, 89% of primary esophageal cancer, and 50% of non-small cell lung cancer. Overexpression of SOX17 in HCT116 CRC cells inhibits colony growth and beta-catenin/T-cell factor-dependent transcription. Structure-based deletion analysis further shows the presence of a Wnt signaling repression domain in the SOX17 HMG box. Together, our studies suggest that SOX17 is a negative modulator of canonical Wnt signaling, and that SOX17 silencing due to promoter hypermethylation is an early event during tumorigenesis and may contribute to aberrant activation of Wnt signaling in CRC.
Figures


References
-
- Gubbay J, Collignon J, Koopman P, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–50. - PubMed
-
- Matsui T, Kanai-Azuma M, Hara K, et al. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119:3513–26. - PubMed
-
- Park KS, Wells JM, Zorn AM, Wert SE, Whitsett JA. Sox17 influences the differentiation of respiratory epithelial cells. Dev Biol. 2006;294:192–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

