Randomized, double-blinded phase II evaluation of docetaxel with or without doxercalciferol in patients with metastatic, androgen-independent prostate cancer
- PMID: 18413835
- PMCID: PMC5648051
- DOI: 10.1158/1078-0432.CCR-07-4274
Randomized, double-blinded phase II evaluation of docetaxel with or without doxercalciferol in patients with metastatic, androgen-independent prostate cancer
Abstract
Purpose: Docetaxel is standard of care for androgen-independent prostate cancer (AIPC). Doxercalciferol (1 alpha-hydroxyvitamin D2) had modest activity in phase I/II trials. Preclinical data support combining vitamin D analogues with docetaxel to treat AIPC.
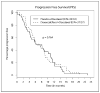
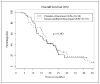
Experimental design: Chemotherapy-naive men with metastatic AIPC were randomized 1:1 to receive, on a 4-week cycle, docetaxel (35 mg/m2 i.v., days 1, 8, and 15) with or without doxercalciferol (10 microg orally, days 1-28). The primary end point was prostate-specific antigen (PSA) response. Secondary end points were progression-free survival, overall survival, objective response, and toxicity. Survival was analyzed as intent to treat.
Results: Seventy patients were randomized. Median follow-up was 17.6 months (range, 3.3-45.2). PSA response rate was 46.7% [95% confidence interval (95% CI), 30-64] in the doxercalciferol arm and 39.4% (95% CI, 25-56) with placebo (P = 0.560). Median progression-free survival in the doxercalciferol arm was 6.17 months (95% CI, 4.20-10.7) versus 6.20 months (95% CI, 4.83-9.07) with placebo (P = 0.764). Median overall survival in the doxercalciferol arm was 17.8 months (95% CI, 14.9-23.6) versus 16.4 months (95% CI, 11.9-23.8) with placebo (P = 0.383). Twenty-four patients in the doxercalciferol arm and 23 in the placebo arm were evaluable for objective response. No complete responses were observed. Partial objective response rate was 12.5% with doxercalciferol versus 8.7% with placebo (P = 0.672). Rate of grade > or =3 toxicity was 46% with doxercalciferol versus 42% with placebo (P = 0.785).
Conclusions: Daily doxercalciferol with weekly docetaxel did not enhance PSA response rate or survival. Toxicity was similar between arms. Despite the disappointing results of this study, other vitamin D analogues remain under active investigation.
Figures
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. - PubMed
-
- Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–11. - PubMed
-
- Schwartz GG, Oeler TA, Uskokovic MR, Bahnson RR. Human prostate cancer cells: inhibition of proliferation by vitamin D analogs. Anticancer Res. 1994;14:1077–81. - PubMed
-
- Skowronski RJ, Peehl DM, Feldman D. Actions of vitamin D3, analogs on human prostate cancer cell lines: comparison with1,25-dihydroxyvitamin D3. Endocrinology. 1995;136:20–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous