Structure determination of a Galectin-3-carbohydrate complex using paramagnetism-based NMR constraints
- PMID: 18413860
- PMCID: PMC2442008
- DOI: 10.1110/ps.034561.108
Structure determination of a Galectin-3-carbohydrate complex using paramagnetism-based NMR constraints
Abstract
The determination of the location and conformation of a natural ligand bound to a protein receptor is often a first step in the rational design of molecules that can modulate receptor function. NMR observables, including NOEs, often provide the basis for these determinations. However, when ligands are carbohydrates, interactions mediated by extensive hydrogen-bonding networks often reduce or eliminate NOEs between ligand and protein protons. In these cases, it is useful to look to other distance- and orientation-dependent observables that can constrain the geometry of ligand-protein complexes. Here we illustrate the use of paramagnetism-based NMR constraints, including pseudo-contact shifts (PCS) and field-induced residual dipolar couplings (RDCs). When a paramagnetic center can be attached to the protein, field-induced RDCs and PCS reflect only bound-state properties of the ligand, even when averages over small fractions of bound states and large fractions of free states are observed. The effects can also be observed over a long range, making it possible to attach a paramagnetic center to a remote part of the protein. The system studied here is a Galectin-3-lactose complex. A lanthanide-binding peptide showing minimal flexibility with respect to the protein was integrated into the C terminus of an expression construct for the Galectin-3-carbohydrate-binding domain. Dysprosium ion, which has a large magnetic susceptibility anisotropy, was complexed to the peptide, making it possible to observe both PCSs and field-induced RDCs for the protein and the ligand. The structure determined from these constraints shows agreement with a crystal structure of a Galectin-3-N-acetyllactosamine complex.
Figures



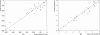
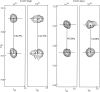

References
-
- Akahani, S., NangiaMakker, P., Inohara, H., Kim, H.R.C., Raz, A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. - PubMed
-
- Al-Hashimi, H.M., Valafar, H., Terrell, M., Zartler, E.R., Eidsness, M.K., Prestegard, J.H. Variation of molecular alignment as a means of resolving orientational ambiguities in protein structures from dipolar couplings. J. Magn. Reson. 2000;143:402–406. - PubMed
-
- Allegrozzi, M., Bertini, I., Janik, M.B.L., Lee, Y.M., Lin, G.H., Luchinat, C. Lanthanide-induced pseudocontact shifts for solution structure refinements of macromolecules in shells up to 40 angstrom from the metal ion. J. Am. Chem. Soc. 2000;122:4154–4161.
-
- Alonso-Plaza, J.M., Canales, M.A., Jimenez, M., Roldan, J.L., Garcia-Herrero, A., Iturrino, L., Asensio, J.L., Canada, F.J., Romero, A., Siebert, H.C., et al. NMR investigations of protein–carbohydrate interactions: Insights into the topology of the bound conformation of a lactose isomer and β-galactosyl xyloses to mistletoe lectin and galectin-1. Biochim. Biophys. Acta. 2001;1568:225–236. - PubMed
-
- Andrec, M., Prestegard, J.H. Metropolis Monte Carlo implementation of Bayesian time-domain parameter estimation: Application to coupling constant estimation from antiphase multiplets. J. Magn. Reson. 1998;130:217–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

